【Domestic Papers】Progress in gallium oxide phosphors: synthesis, characteristics, and versatile application
日期:2025-12-11阅读:28
Researchers from the Zhejiang Normal University have published a dissertation titled "Progress in gallium oxide phosphors: synthesis, characteristics, and versatile application" in Inorganic Chemistry Communications.
Project Support
The authors thank Zhejiang Normal University, Jinhua, China, for providing funds and infrastructural support. They also express sincere gratitude to Harikrishnan Venkatesvaran, National Taipei University of Technology, Taiwan, for sketching the graphical abstract. This was also supported by the GLAMP program of the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (RS2024-00444460).
Background
Phosphor materials are characterised by their ability to emit visible light when stimulated by electromagnetic radiation. They have been extensively studied over the past several decades due to their numerous applications in sensors, lighting, imaging systems, and display technologies. The phosphor with a wide bandgap has garnered significant attention in optoelectronic devices. Notably, these materials, such as GaN and ZnO, have emerged as prominent host matrices due to their large band gaps, suitable carrier paths, and high thermal and chemical stability.
In recent years, Ga2O3 (4.9 eV) has emerged as a promising host material due to its wider bandgap compared to GaN (~3.4 eV) and ZnO (~3.37 eV). Ga2O3 typically exists in five distinct crystallographic phases: α, β, γ, δ, and ε. Among these, β-Ga2O3 has been the most widely studied due to its excellent phase stability, minimal thermal quenching, strong radiation hardness, and superior luminescence performance. Furthermore, its remarkable thermal stability and high melting point of 1800 °C make β-Ga2O3 suitable for a wide range of optoelectronic applications, both at low and high temperatures.
Abstract
This review article offers a comprehensive overview of the latest advancements in gallate phosphors, highlighting their unique properties and diverse applications. The study explores the fundamental mechanisms of phosphors, particularly focusing on gallate-based systems, and evaluates various synthesis methods used to develop these materials. The article discusses the influence of morphology and crystal structure on emission performance, clarifying how specific structural configurations enhance luminescence efficiency. It details essential luminescence metrics, such as quantum yield, thermal stability, lifetime, and color purity, providing insights into performance and optimisation. Furthermore, the article emphasises the significance of gallate phosphors in diverse applications, including bioimaging, horticulture, supercapacitors, stress sensing, photocatalysis, anti-counterfeiting, LEDs, and thermometry. This article aims to serve as a valuable resource for understanding the current state of gallate phosphor technology and its future potential, drawing on recent research findings and developments.
Highlights
● Gallate Phosphor Fundamentals– Explores the structural and optical mechanisms behind gallate-based phosphors, linking crystal chemistry to luminescence.
● Synthesis & Optimization – Reviews various preparation methods (solid-state, sol-gel, hydrothermal) and their effects on phosphor performance.
● Key Performance Metrics– Analyzes quantum yield, thermal stability, lifetime, and color purity to guide material design for enhanced efficiency.
● Diverse Applications – Highlights uses in LEDs, bioimaging, anti-counterfeiting, stress sensing, and photocatalysis, showcasing versatility.
● Future Challenges & Opportunities– Identifies research gaps and potential advancements for next-generation gallate phosphors in emerging technologies.
Conclusion
Gallate phosphors are versatile luminescent materials known for their tunable emission properties, stability, and adaptable crystal structures, making them valuable in various scientific and technological applications. Their synthesis is achieved through methods such as solid-state reactions, sol-gel techniques, hydrothermal processes, combustion, and liquid-phase techniques, allowing for precise control over particle morphology, crystallinity, and optical performance. Doping with rare-earth and transition-metal ions enables a broad spectrum of colors, enhancing their appeal for display technologies, lighting, bioimaging, and sensors.
Structurally, gallate phosphors with spinel, garnet, and perovskite frameworks provide stable lattice environments that enhance luminescence efficiency, thermal stability, and dopant integration—factors crucial for high -performance devices. Rare-earth-doped gallate phosphors demonstrate superior biocompatibility and deeper tissue penetration through near -infrared emissions, thus expanding their use in clinical diagnostics. Their temperature -sensitive luminescence makes them effective for non -contact temperature assessments in harsh environments. Additionally, their mechanoluminescent properties support stress -sensing technologies, which are beneficial for structural health monitoring in civil and aerospace engineering. They also promote crop growth in horticulture by converting ultraviolet radiation to photosynthetically active radiation.
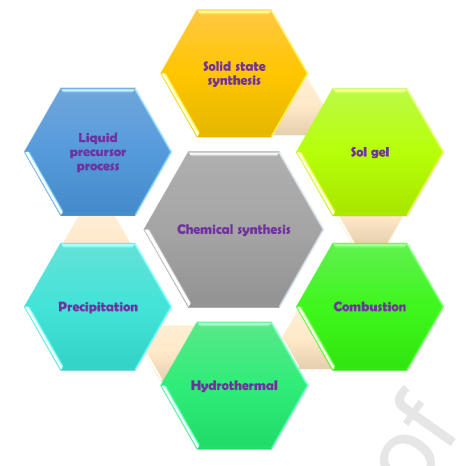
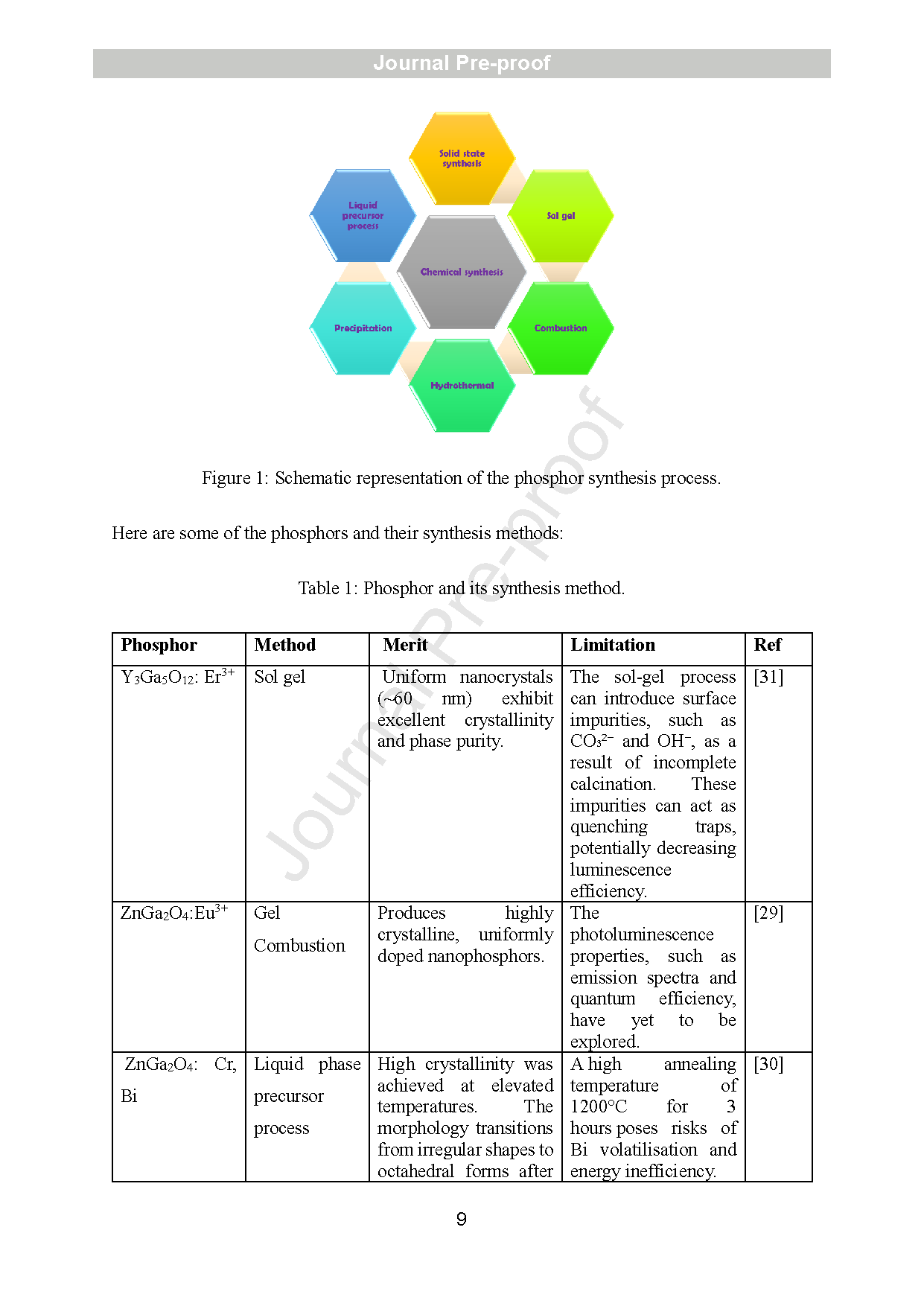
Figure 1: Schematic representation of the phosphor synthesis process.
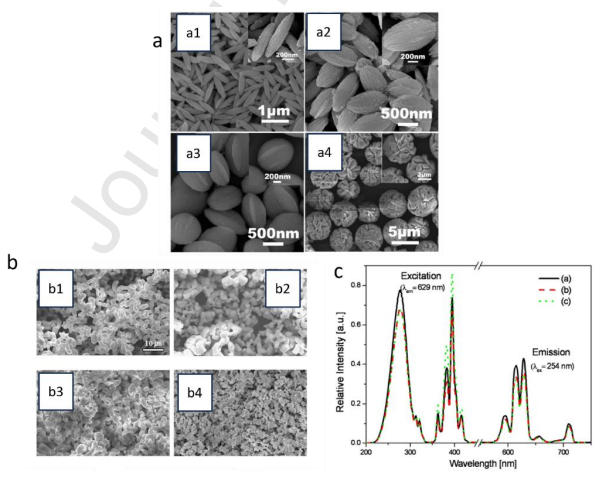
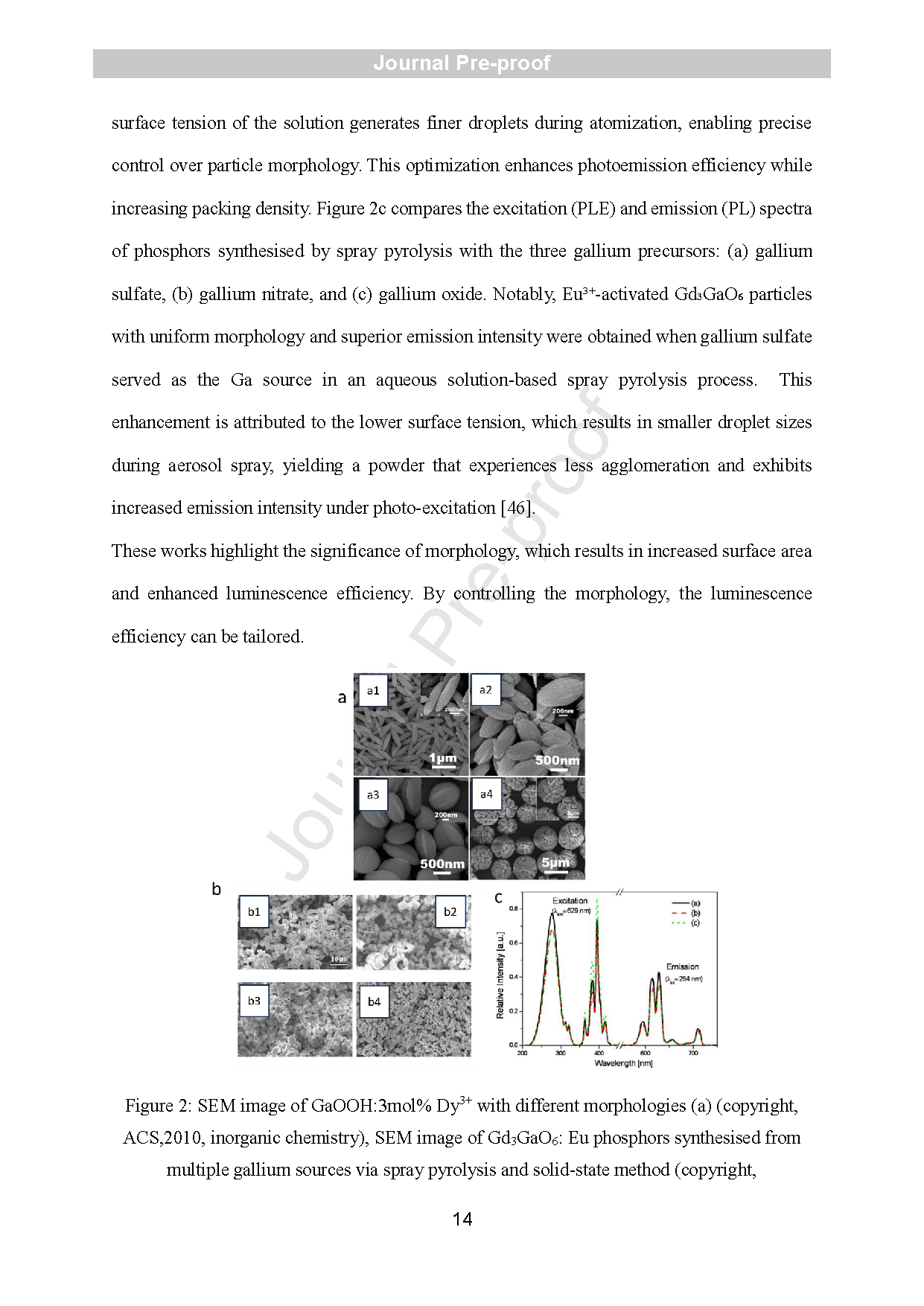
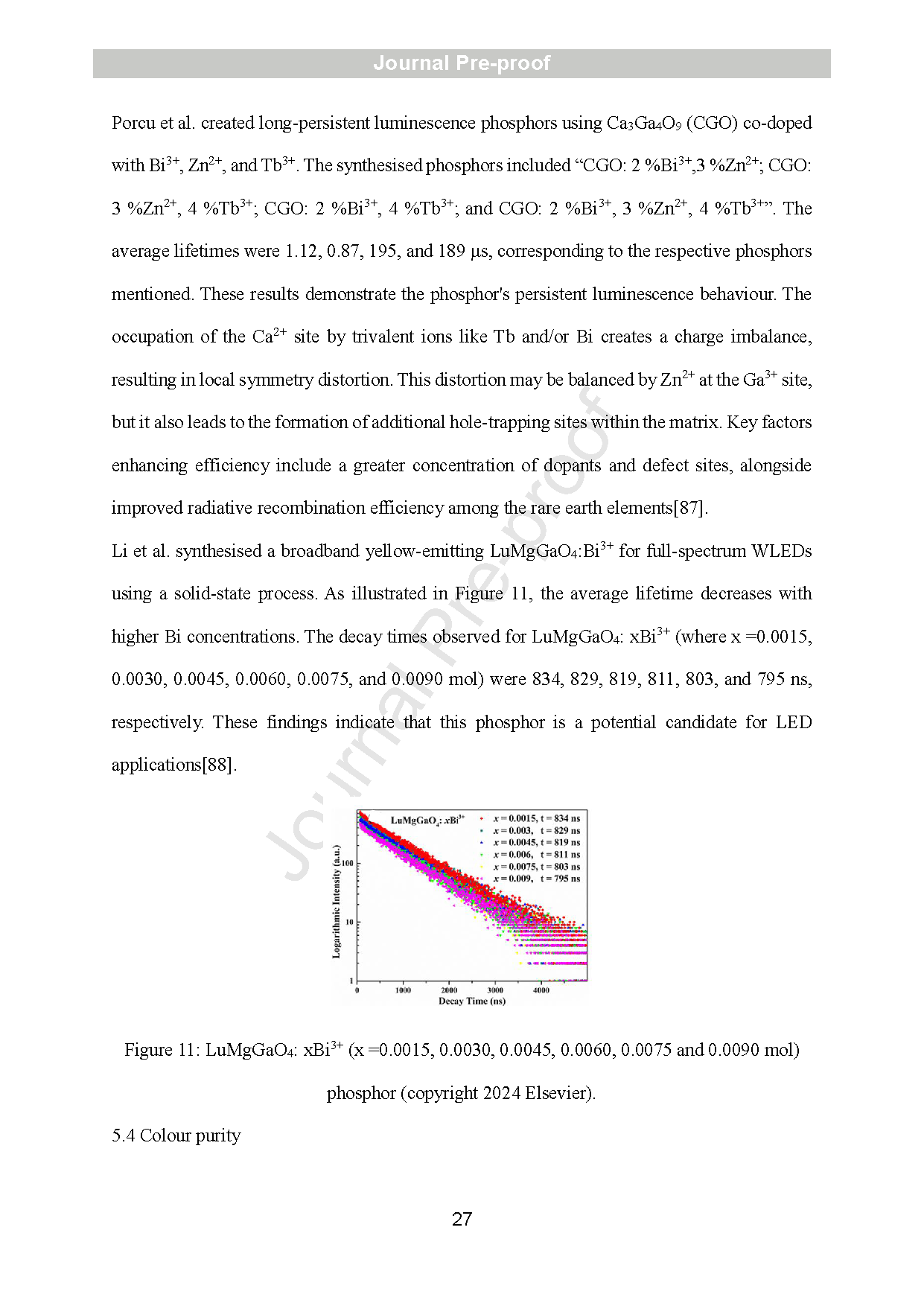
Figure 2: SEM image of GaOOH:3mol% Dy3+ with different morphologies (a) (copyright, ACS,2010, inorganic chemistry), SEM image of Gd3GaO6: Eu phosphors synthesised from multiple gallium sources via spray pyrolysis and solid -state method (copyright, Elsevier,2009, optical materials) (b). Comparative PLE and PL spectra of Gd3GaO6:Eu phosphors with varying Ga sources (c) (copyright Elsevier, 2009).
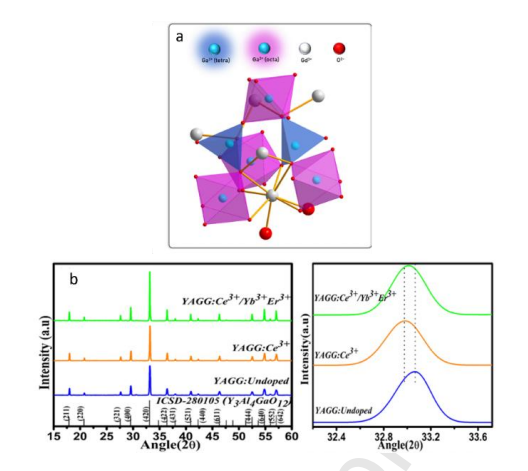
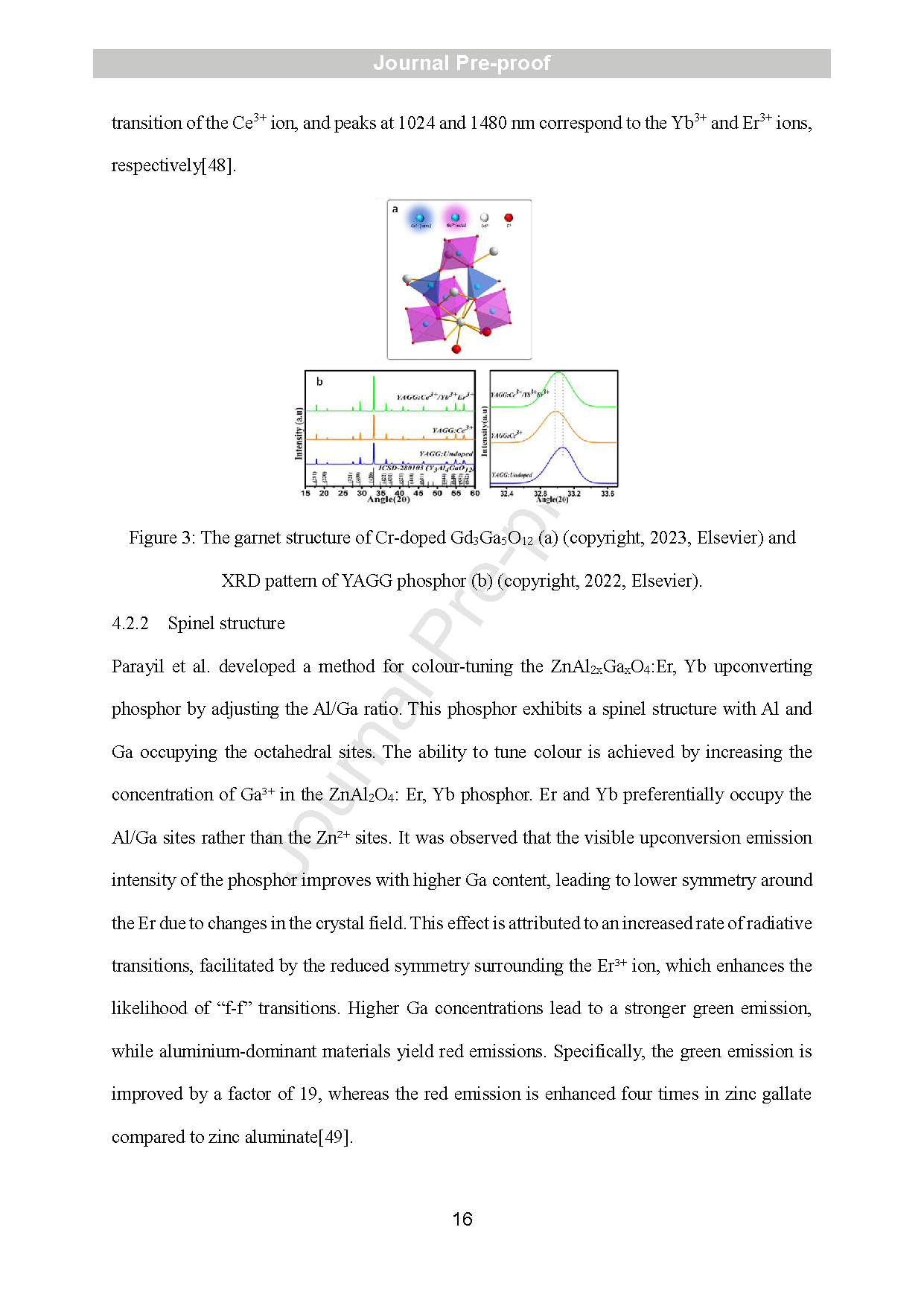
Figure 3: The garnet structure of Cr -doped Gd3Ga5O12 (a) (copyright, 2023, Elsevier) and XRD pattern of YAGG phosphor (b) (copyright, 2022, Elsevier) .
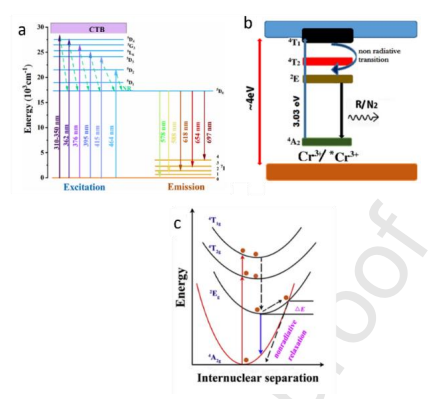
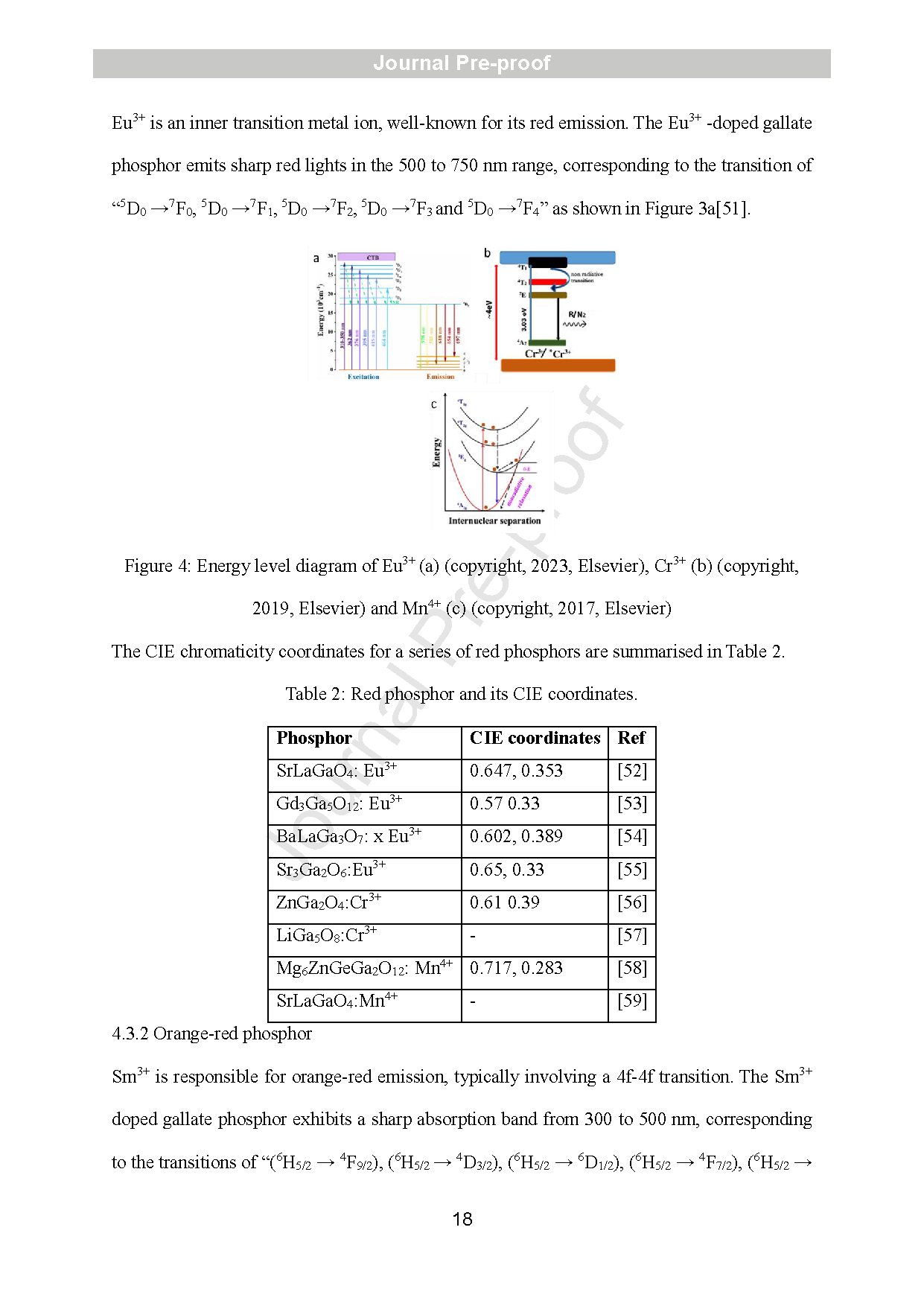
Figure 4: Energy level diagram of Eu3+ (a) (copyright, 2023, Elsevier), Cr3+ (b) (copyright, 2019, Elsevier) and Mn4+ (c) (copyright, 2017, Elsevier)
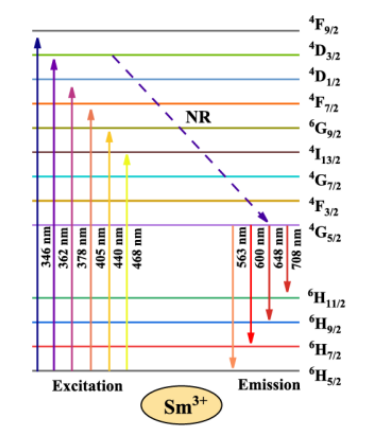
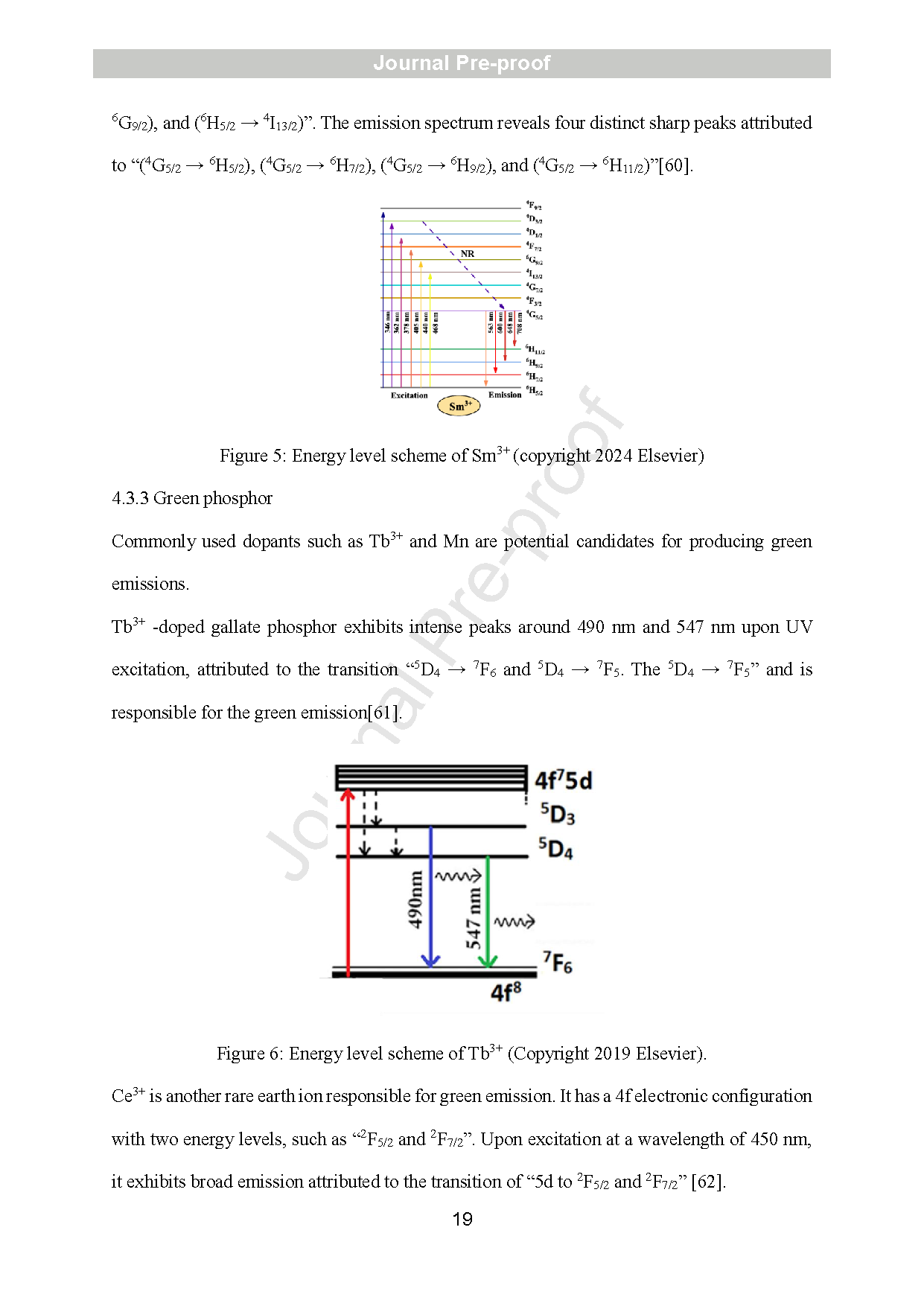
Figure 5: Energy level scheme of Sm3+ (copyright 2024 Elsevier)
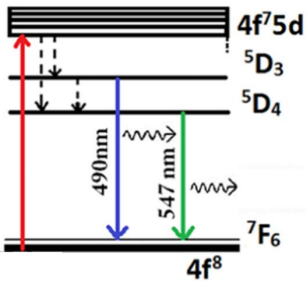
Figure 6: Energy level scheme of Tb3+ (Copyright 2019 Elsevier).
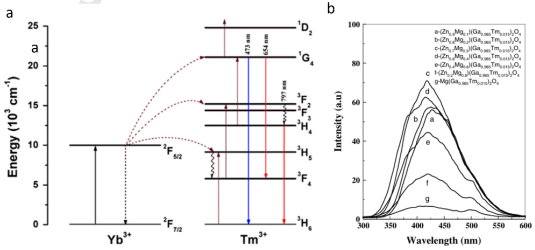
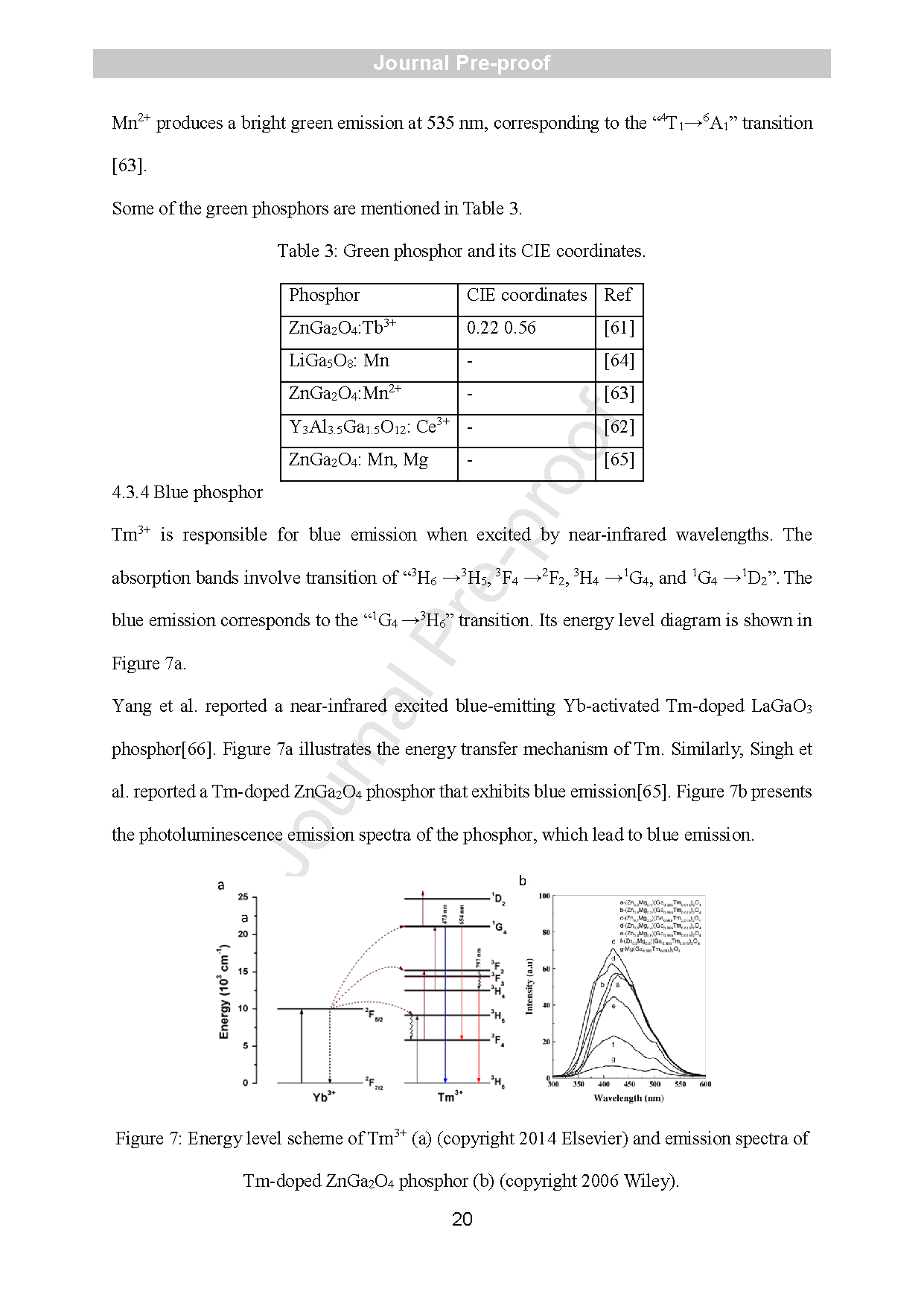
Figure 7: Energy level scheme of Tm3+ (a) (copyright 2014 Elsevier) and emission spectra of Tm -doped ZnGa2O4 phosphor (b) (copyright 2006 Wiley).
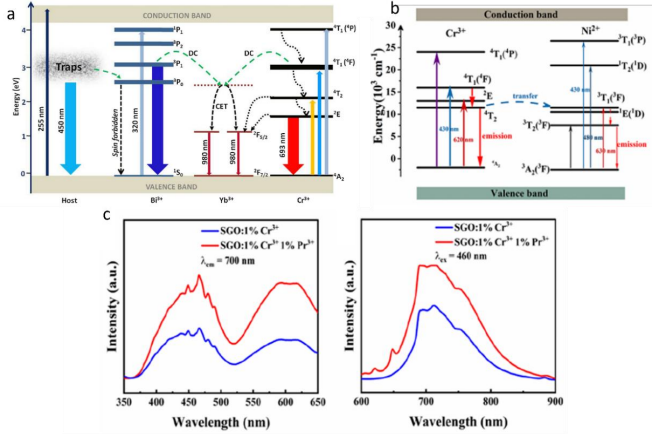
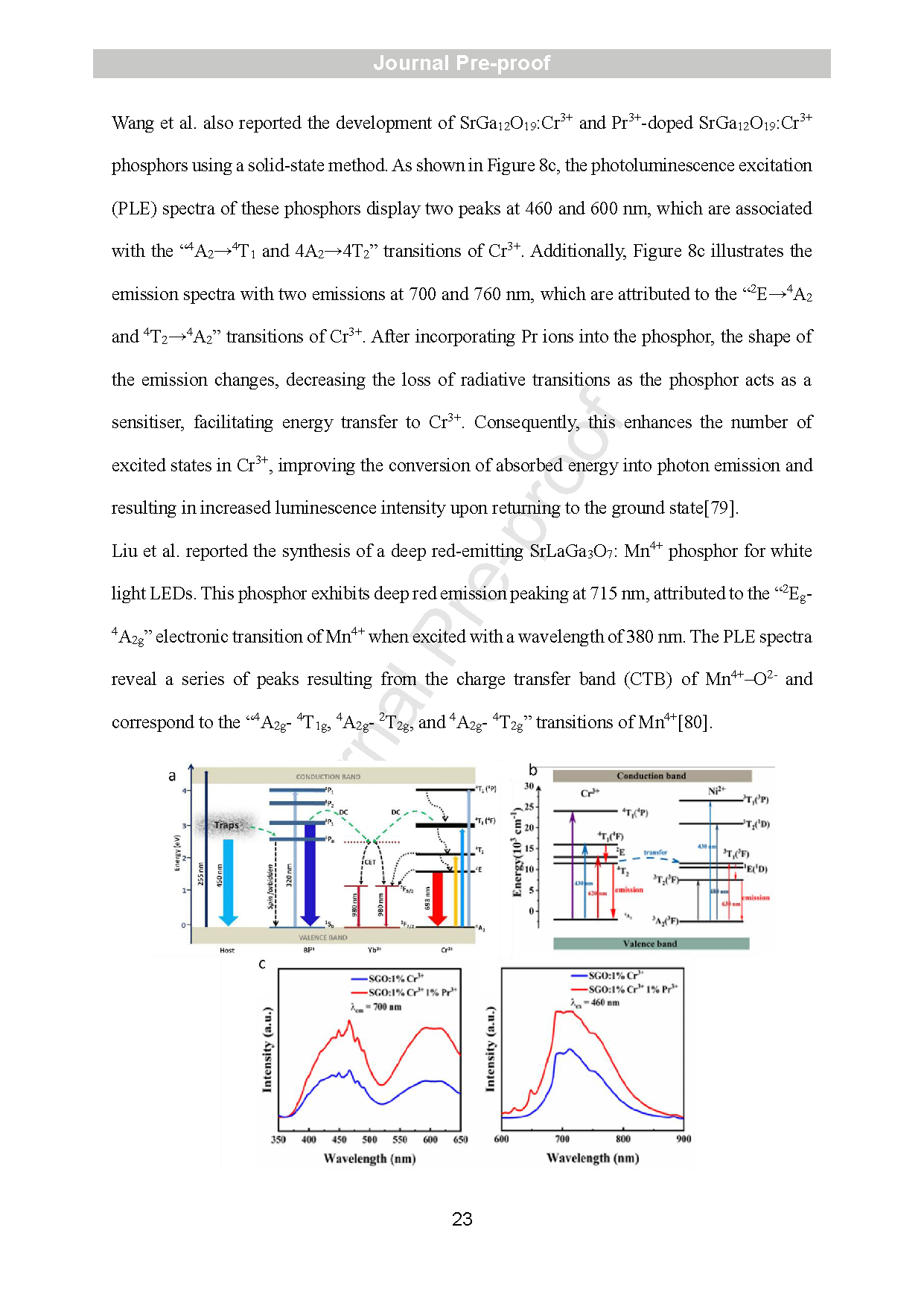
Figure 8: Energy transfer mechanism of Bi3+/Cr3+/Yb3+ doped in CaGa2O4 (a) (copyright 2018 Elsevier), and Mg3Ga2GeO8: Cr3+, Ni2+ (b) (copyright 2019 Elsevier). PLE and PL of SrGa12O19:Cr3+&Pr3+-doped SrGa12O19: Cr3+ (c) (copyright 2024 ACS) phosphor.
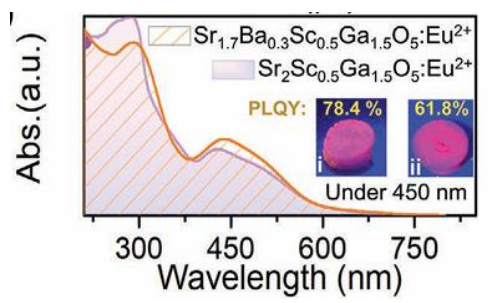
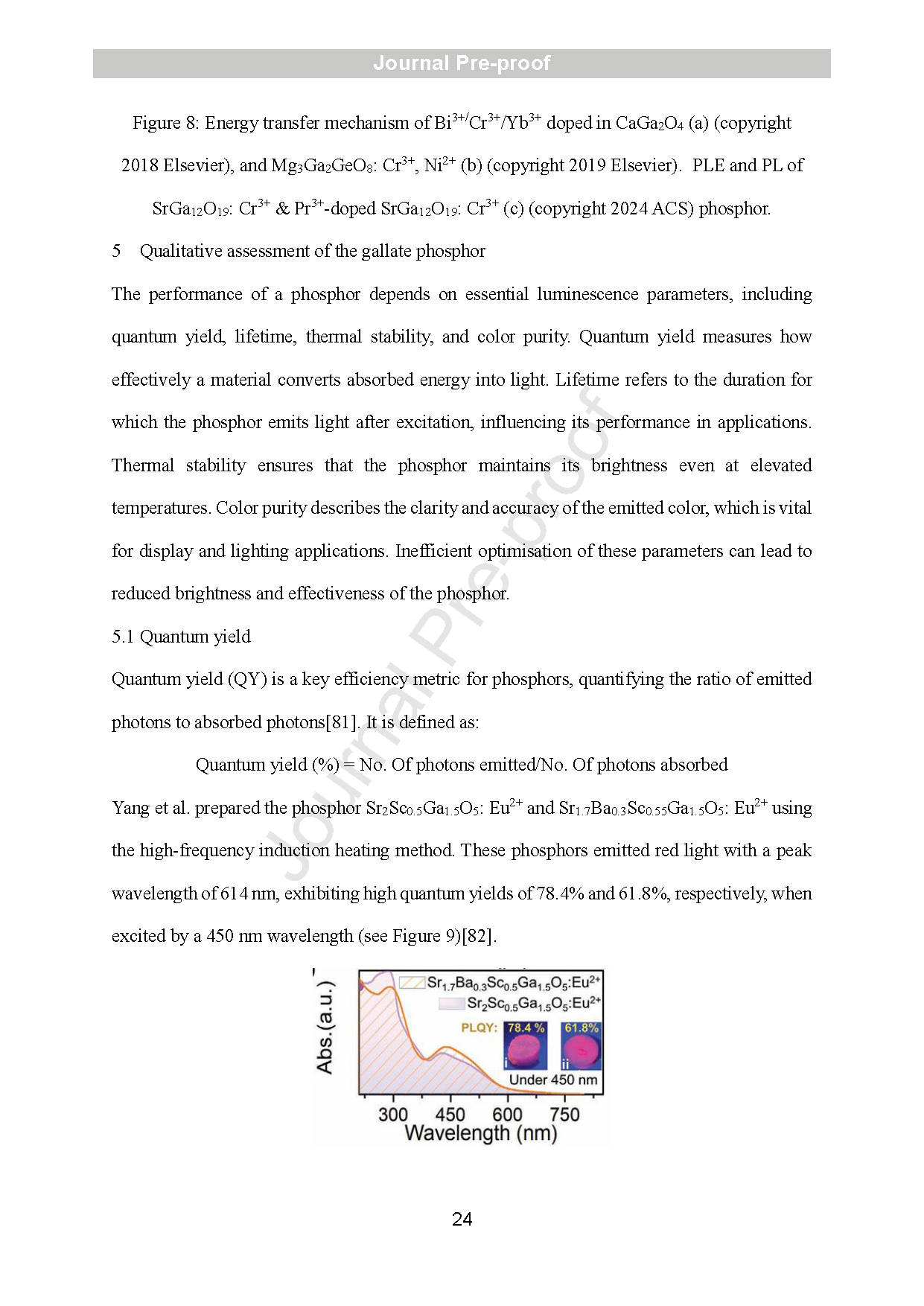
Figure 9: Absorption spectra of Sr2Sc0.5Ga1.5O5: Eu2+ and Sr1.7Ba0.3Sc0.55Ga1.5O5: Eu2+ phosphors (copyrights 202 1 Wiley).
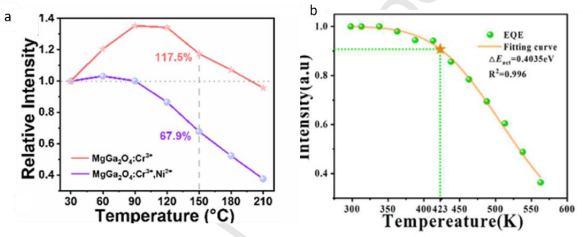
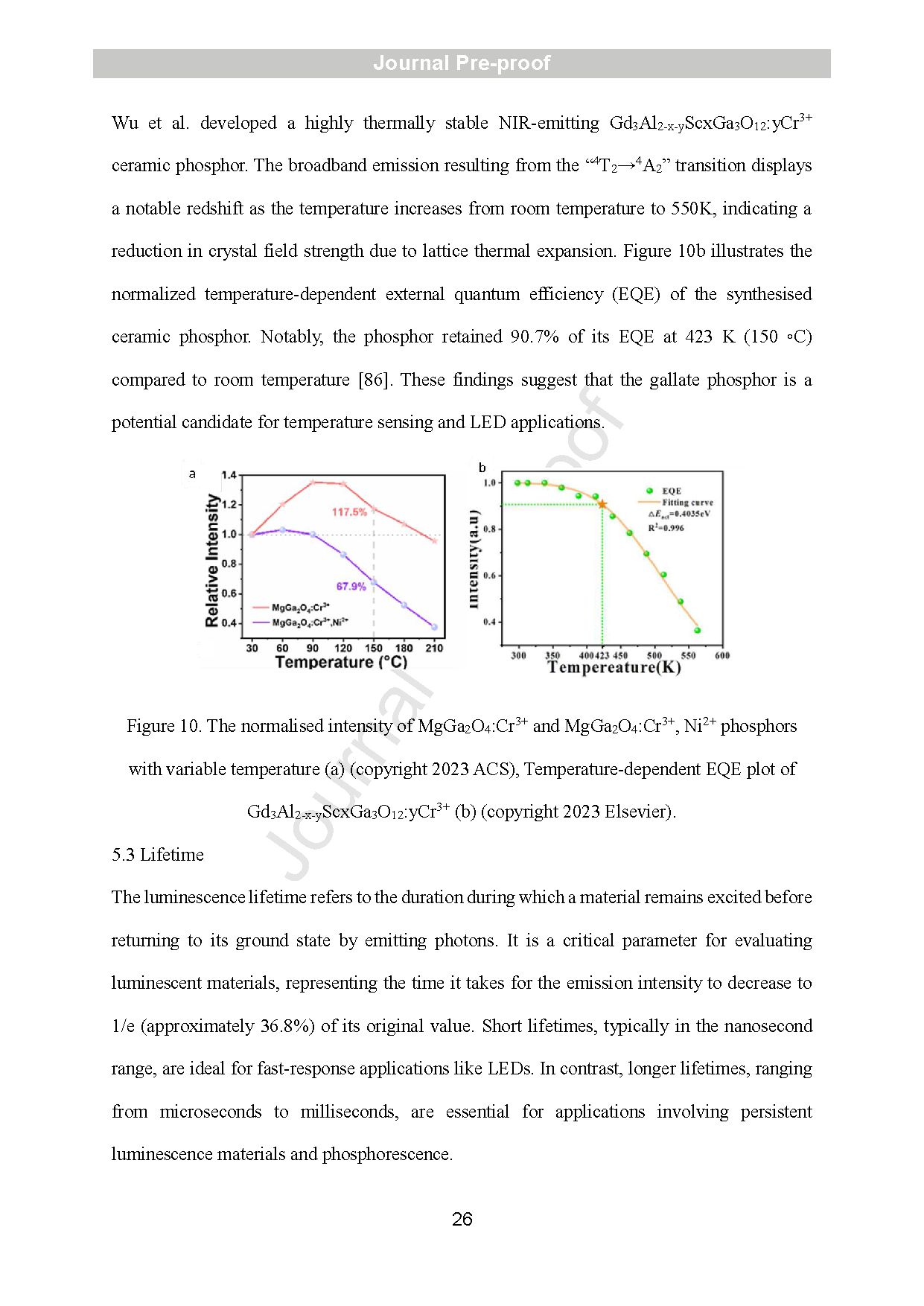
Figure 10. The normalised intensity of MgGa2O4:Cr3+ and MgGa2O4:Cr3+, Ni2+ phosphors with variable temperature (a) (copyright 2023 ACS), Temperature-dependent EQE plot of Gd3Al2-x-yScxGa3O12:yCr3+ (b) (copyright 2023 Elsevier).
DOI:
doi.org/10.1016/j.inoche.2025.115973