【International Papers】Gas-sensitive properties of PECVD nanocrystalline Ga₂O₃ thin films doped with Zn
日期:2025-09-25阅读:49
Researchers from the National Research Tomsk State University have published a dissertation titled "Gas-sensitive properties of PECVD nanocrystalline Ga2O3 thin films doped with Zn" in Results in Surfaces and Interfaces.
Background
Gallium oxide (Ga2O3) has attracted considerable attention as a promising material for power electronics and gas sensors due to its outstanding fundamental properties, which even surpass those of well-established SiC and GaN. Gas sensors based on Ga2O3 exhibit high sensitivity to various gases over a wide temperature range (from room temperature to 1000 °C) and demonstrate excellent chemical and thermal stability. However, pure β-Ga2O3 thin films show limited gas sensitivity, so it is often necessary to enhance their response by introducing dopants or additives of other semiconductors with smaller bandgaps and catalytic activity, such as SnO2, In2O3, or ZnO. ZnO is a widely studied semiconductor for gas sensors, known for its high chemical and thermal stability, and is commonly used to modify other metal oxides to improve gas-sensing performance. Previous studies have focused on Ga-doped ZnO thin films, which exhibit high gas sensitivity, whereas research on Zn-doped Ga2O3 (ZGO) remains limited. Existing reports indicate that ZGO can achieve high responses to O2 at moderate operating temperatures (400–500 °C). Metal oxide thin films used in gas sensors are typically polycrystalline, and the grain size has a significant effect on gas-sensing performance; films with nanocrystalline grains (10–100 nm) often show enhanced sensitivity. Therefore, nanocrystalline Zn-doped Ga2O3 (nc-ZGO) thin films represent a promising material for high-performance sensor layers. In this study, nc-ZGO thin films were deposited via PECVD, and their gas-sensitive properties were systematically investigated for the first time, providing a foundation for the development of high-performance gas sensors.
Abstract
Nanocrystalline Ga2O3 thin films doped by Zn (nc-ZGO) were synthesized by plasma-enhanced chemical vapor deposition. Their gas-sensitive properties have been comprehensively investigated. The thin films exhibit high response and operation speed under exposure to industry-relevant gases such as CO, H2, NH3 and NO2. The highest responses are achieved under H2 exposure. The responses to 500 ppm and 104 ppm of H2 are 2.99 a. u. and 24.5 a. u., respectively, at the maximum response temperature of 500 °C. The sum of the response and recovery times do not exceed 27.4 s. The nc-ZGO thin films show only a weak drift of their gas-sensitive characteristics during long-term exposure to CO, H2, and NO2 as well as and a weak dependence of the gas-sensitive characteristics on the humidity level in the 10–50 % relative humidity range. A mechanism of the sensing effect of nc-ZGO thin films is proposed within the current paradigm.
Highlights
● Nanocrystalline Ga2O3films heavily doped with Zn (ZGO) were synthesized by PECVD.
● ZGO films surface morphology is characterized by grains with a size of 12–25 nm.
● Responses to 500 ppm and 104ppm of H2are 2.99 and 24.5 at 500 °C.
● Response and recovery times in sum do not exceed 27.4 s at 500 °C.
● ZGO films demonstrate long-term stability of the gas-sensitive characteristics.
Conclusion
The gas-sensitive properties of nanocrystalline Zn-doped PECVD deposited Ga2O3 thin films to a number of habitat and industry-relevant gases have been comprehensively studied. The concentration of Zn in the films is 1.64 at%, the impurity homogeneously distributed over the area of nanocrystalline Ga2O3 thin films. The surface morphology of Zn-doped nanocrystalline Ga2O3 thin films is presented by grains with the size of 12–25 nm. The samples are characterized by high transmittance, which reaches almost 90 % at λ = 450 nm. The Zn doped nanocrystalline Ga2O3 thin films exhibit high response and speed performance under exposure to CO, H2, NH3, and NO2. The highest responses were achieved under exposure to H2. At the maximum response temperature corresponding to 500 °C, the responses to 500 ppm and 104 ppm of H2 were 2.99 a. u. and 24.5 a. u., respectively. The sum of the response and recovery times did not exceed 27.4 s. Compared to sensors based on nanocrystalline films of other metal oxides, the feature of the sensors based on Zn-doped nanocrystalline Ga2O3 thin films is a weak drift of gas-sensitive characteristics during long-term tests under exposure to CO, H2, and NO2, at relatively high operating temperatures and small grain sizes. In addition, the samples are characterized by a weak dependence of gas-sensitive characteristics on humidity in the relative humidity range of 10–50 %. Within the current paradigm, the mechanism of the sensing effect of Zn doped nanocrystalline Ga2O3 thin films is proposed. The gas-sensitive characteristics achieved for sensors based on Zn doped nanocrystalline Ga2O3 thin films indicate their potential for detecting pre-explosive H2 concentrations in extreme operating conditions and ensuring safety in nuclear and hydrogen energy applications.
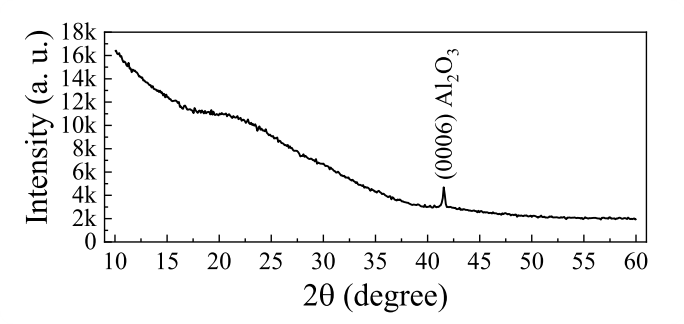
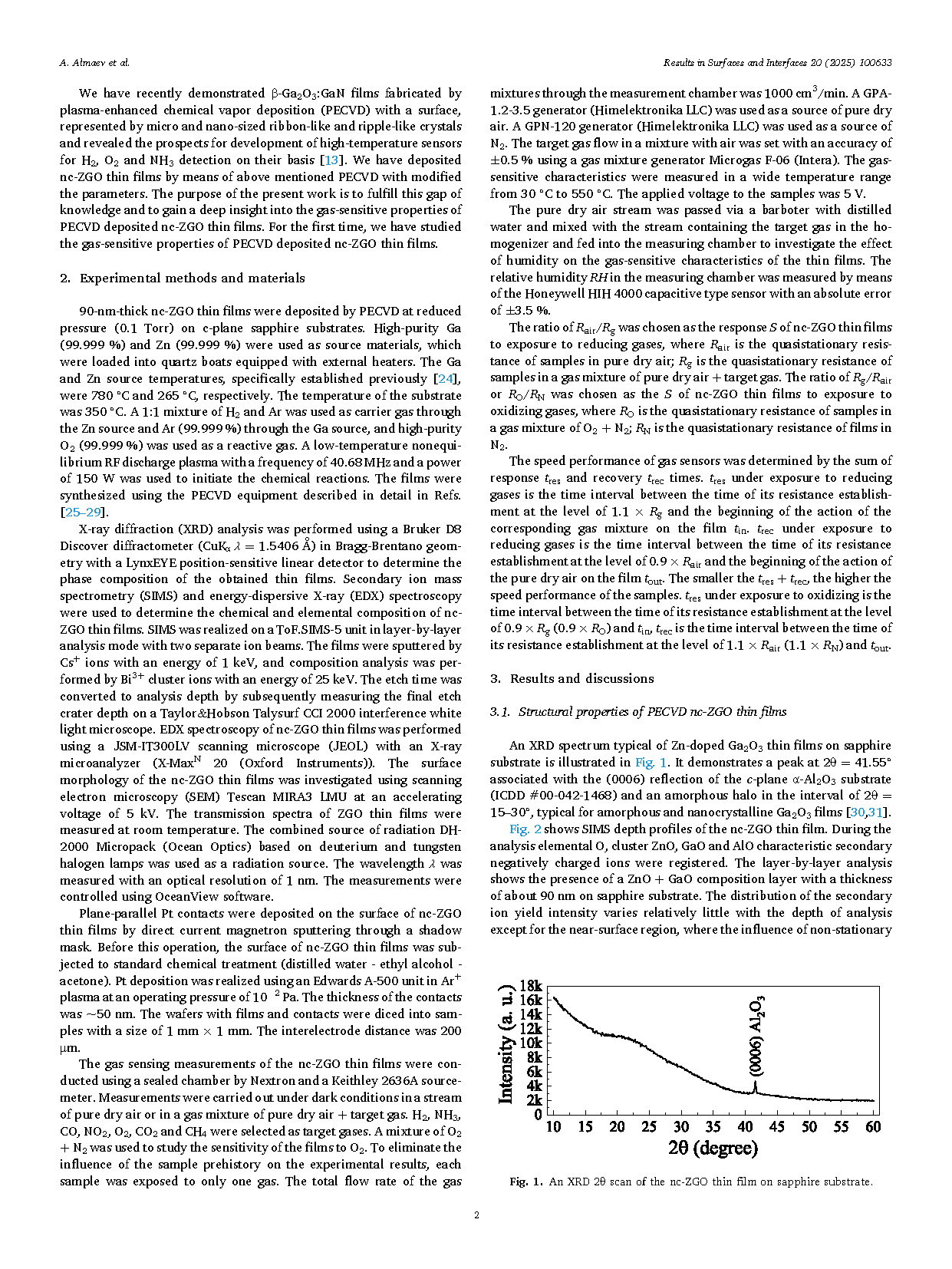
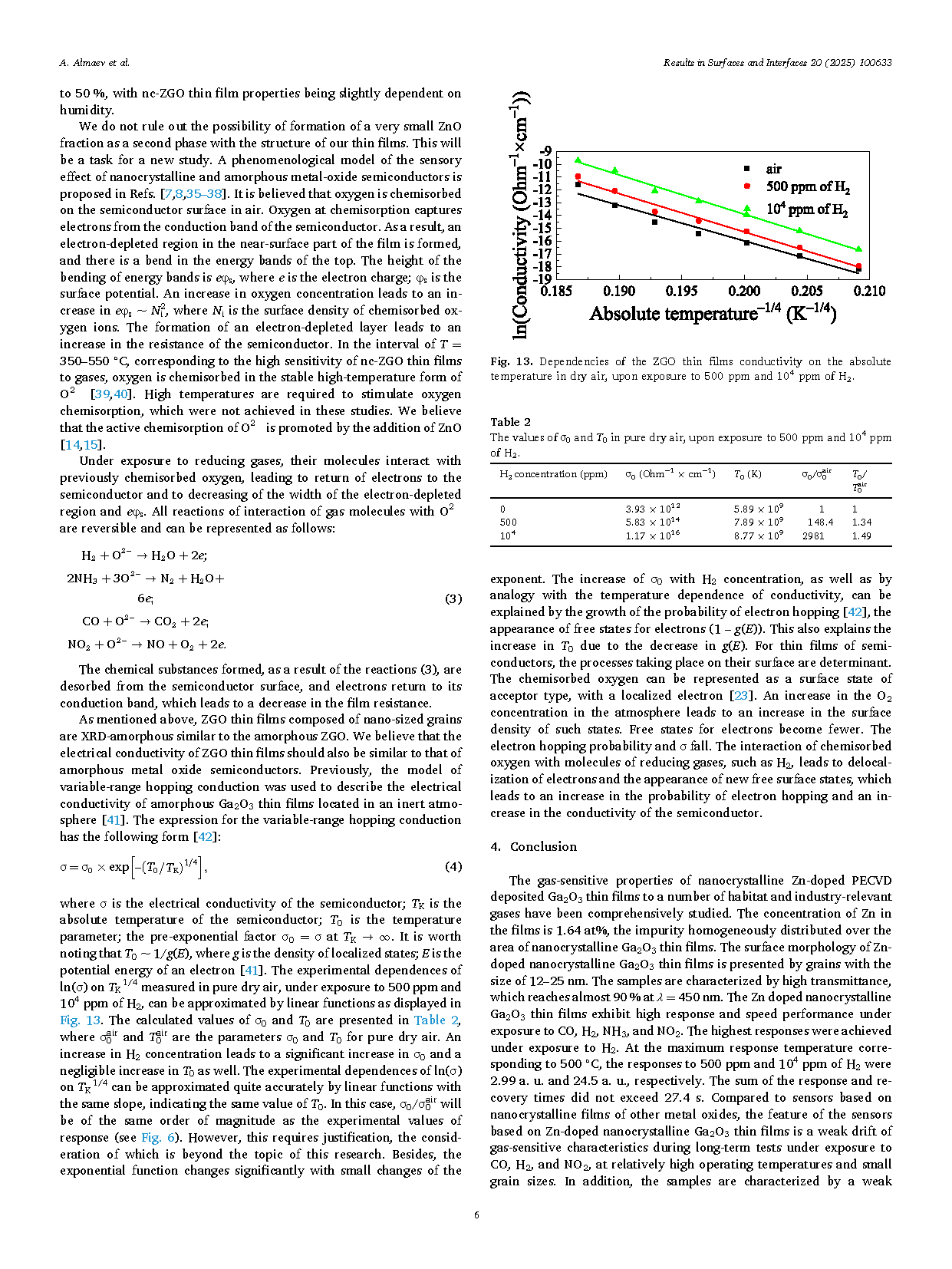
Fig. 1. An XRD 2θ scan of the nc-ZGO thin film on sapphire substrate.
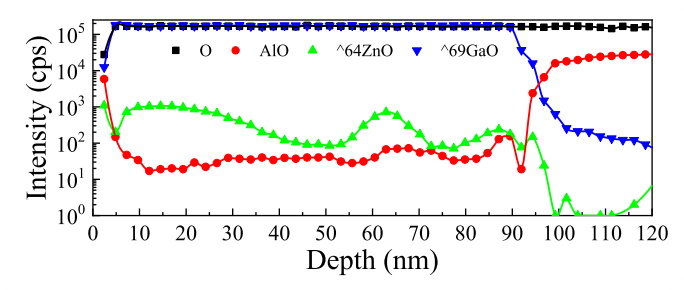
Fig. 2. SIMS in-depth profile of the nc-ZGO thin film on sapphire substrate.
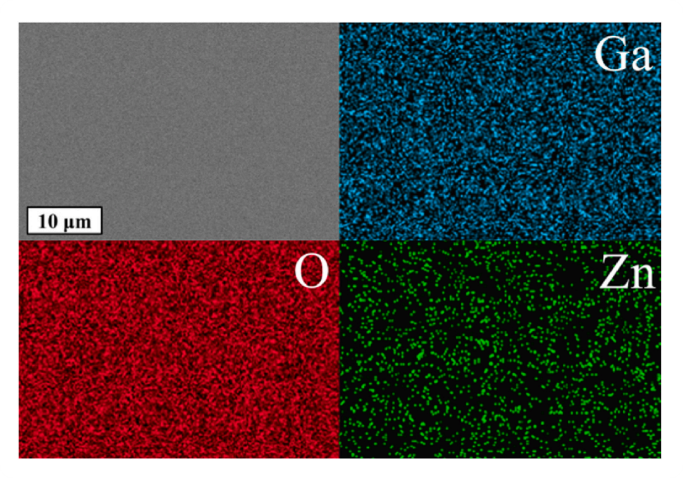
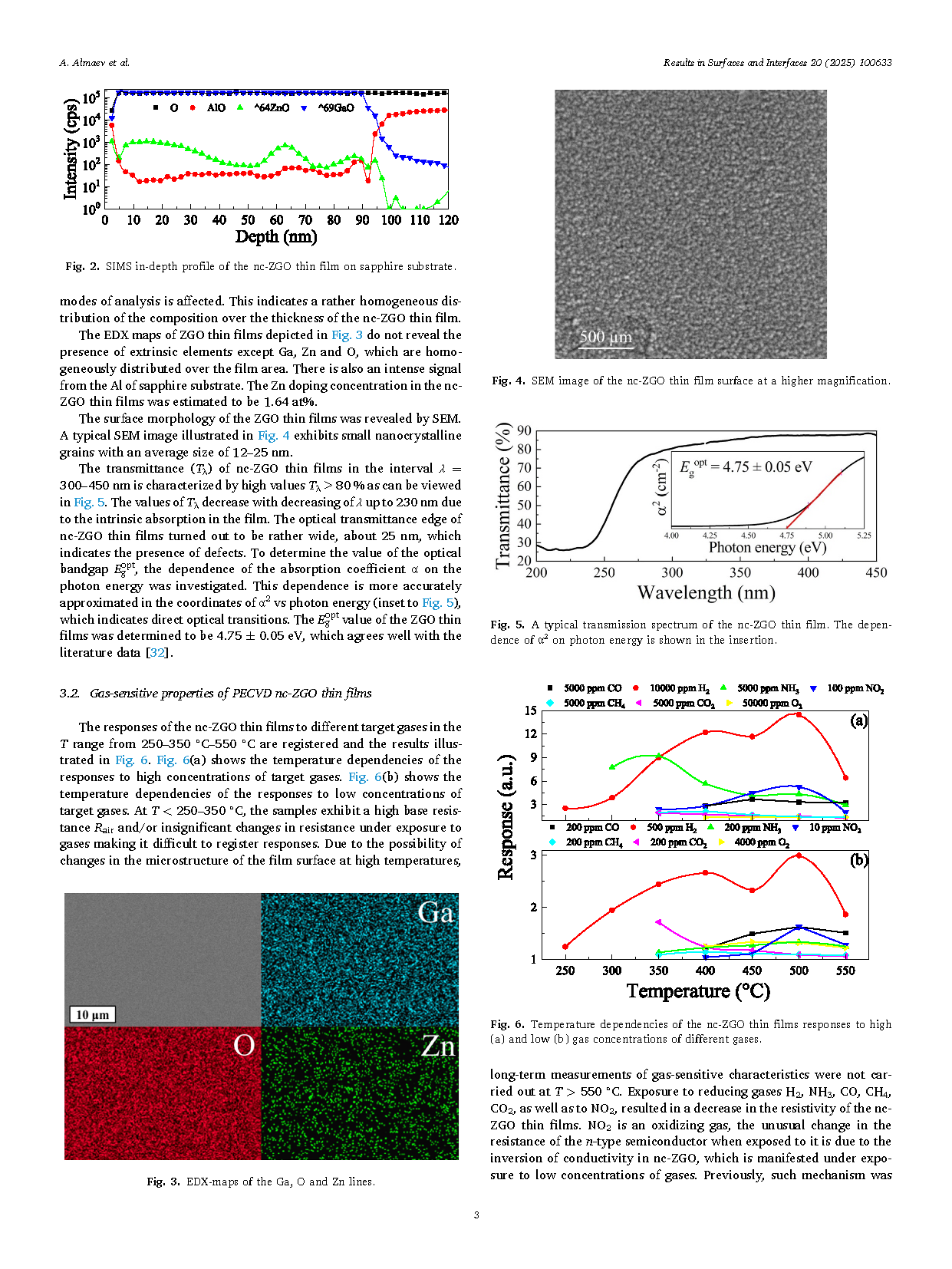
Fig. 3. EDX-maps of the Ga, O and Zn lines.
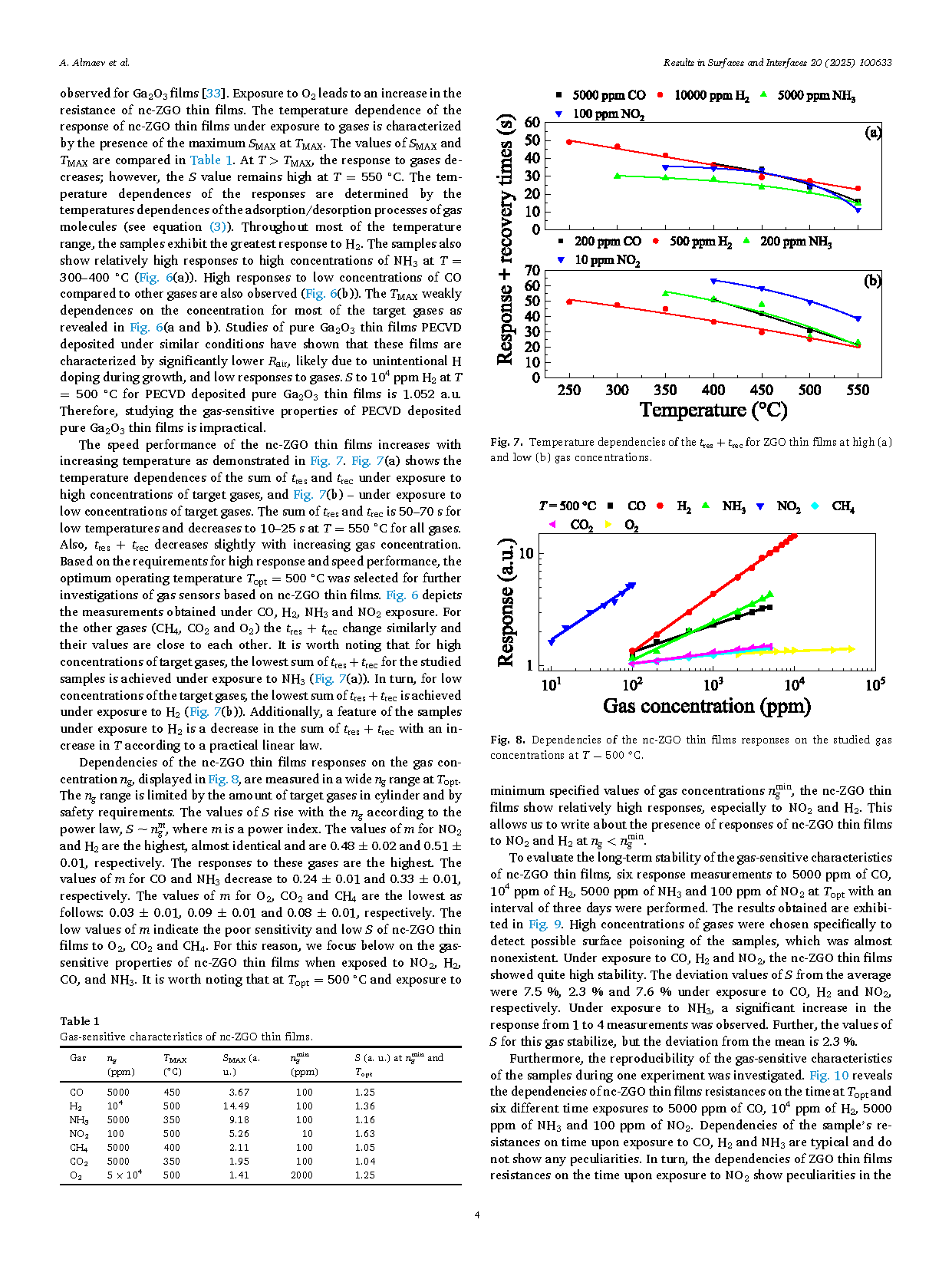
Fig. 4. SEM image of the nc-ZGO thin film surface at a higher magnification.
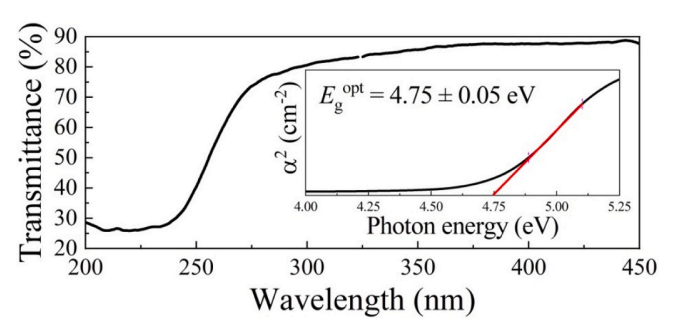
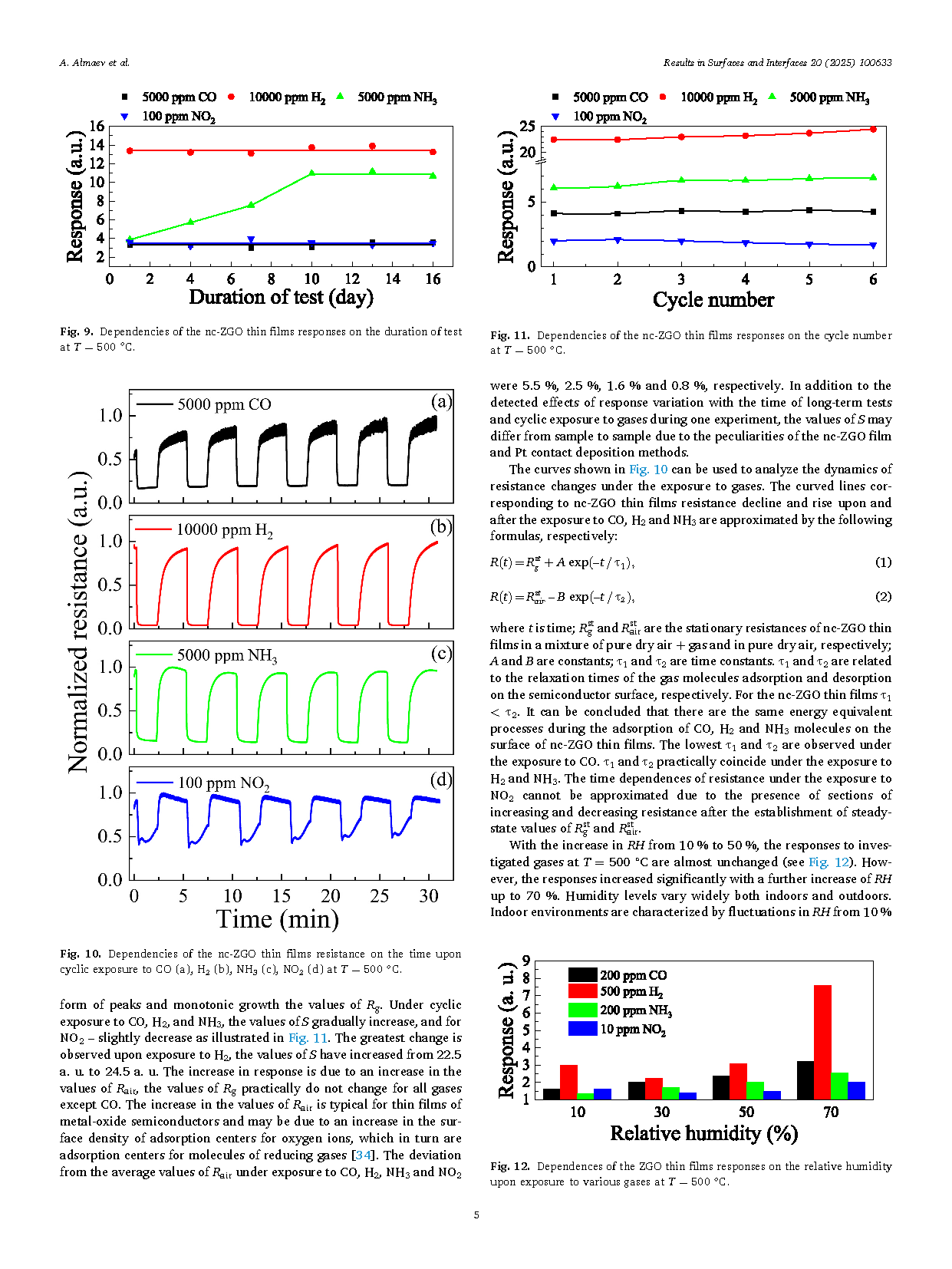
Fig. 5. A typical transmission spectrum of the nc-ZGO thin film. The dependence of α2 on photon energy is shown in the insertion.
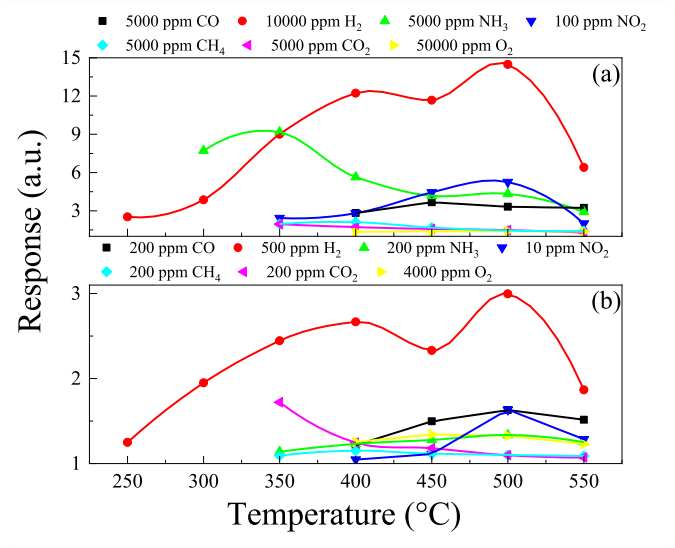
Fig. 6. Temperature dependencies of the nc-ZGO thin films responses to high and low (b) gas concentrations of different gases.
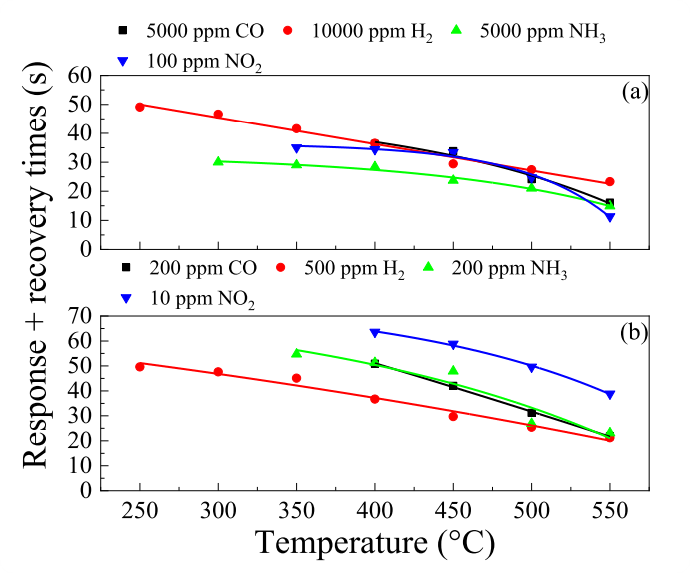
Fig. 7. Temperature dependencies of the tres + trec for ZGO thin films at high (a) and low (b) gas concentrations
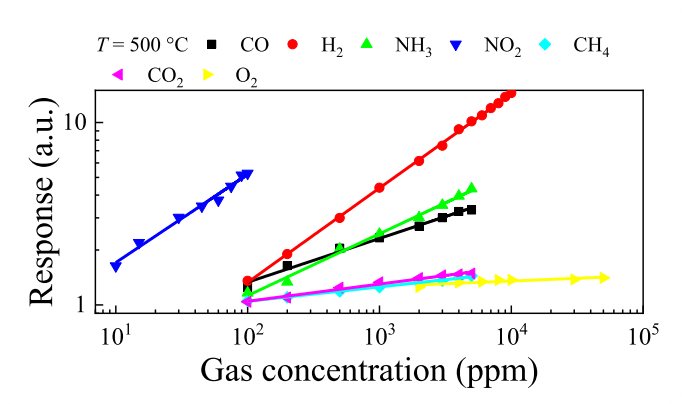
Fig. 8. Dependencies of the nc-ZGO thin films responses on the studied gas concentrations at T = 500 ℃.
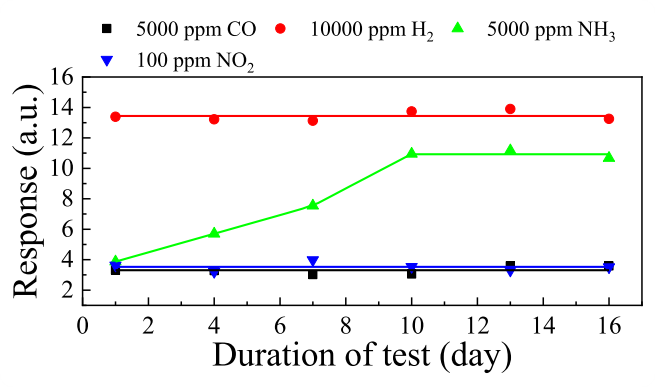
Fig. 9. Dependencies of the nc-ZGO thin films responses on the duration of test at T = 500 ℃.
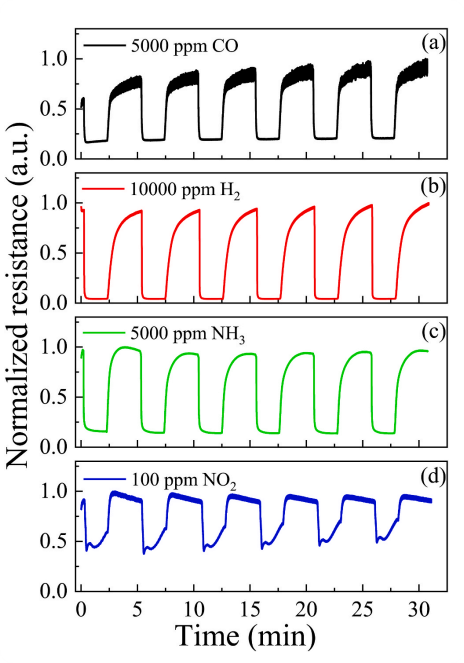
Fig. 10. Dependencies of the nc-ZGO thin films resistance on the time upon cyclic exposure to CO (a), H2 (b), NH3 (c), NO2 (d) at T = 500 ℃.
DOI:
doi.org/10.1016/j.rsurfi.2025.100633