【Domestic Papers】Inner Mongolia University---Study on crystallization and oxygen vacancies of β-Ga₂O₃ powder synthesized from various alkalis
日期:2025-05-27阅读:360
Researchers from the Inner Mongolia University have published a dissertation titled "Study on crystallization and oxygen vacancies of β-Ga2O3 powder synthesized from various alkalis" in Journal of Alloys and Compounds.
Project Support
This work was supported by the National Natural Science Foundation of China (NNSFC) (Grant Nos. 62364014 and 12164031).
Background
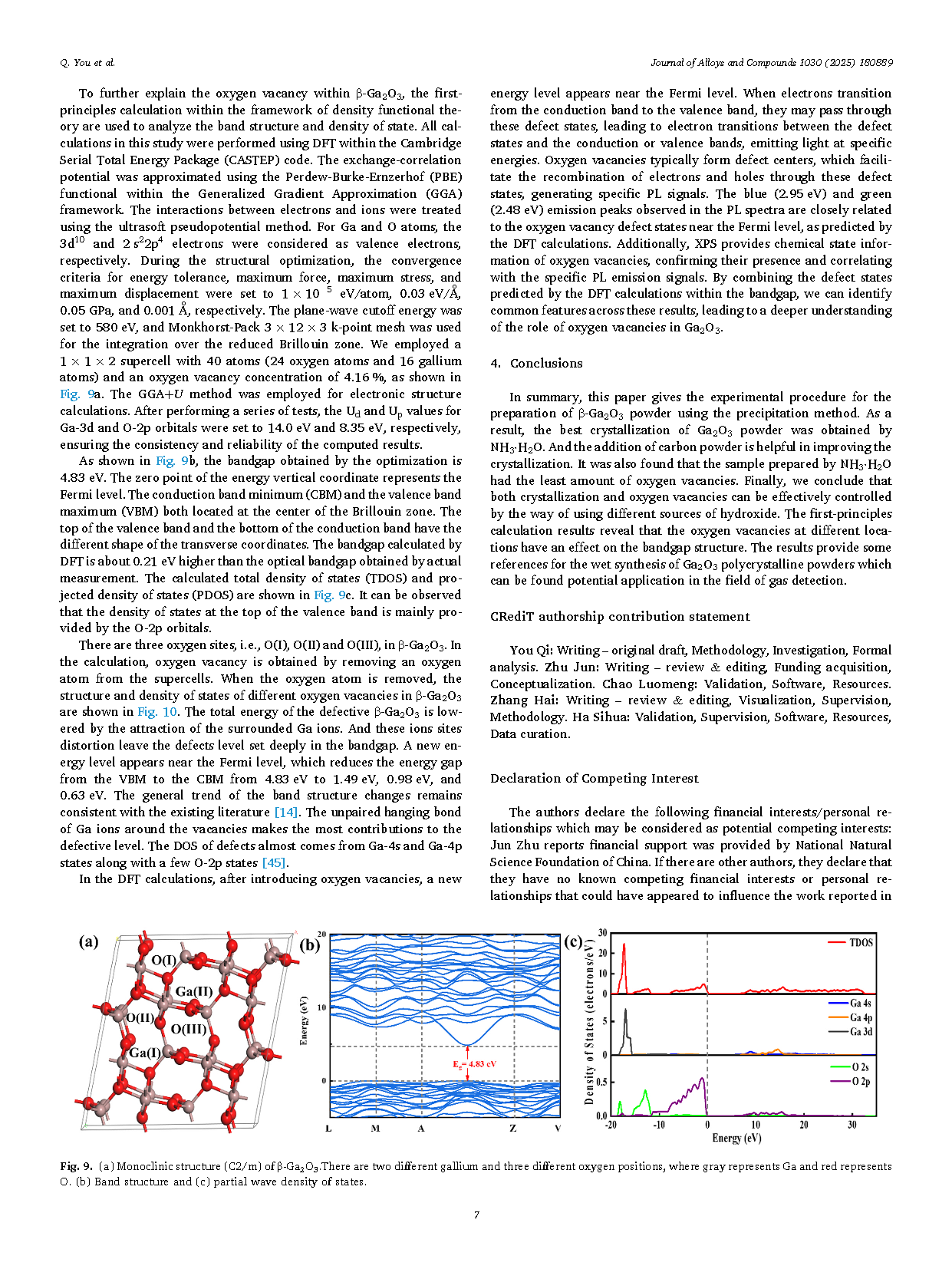
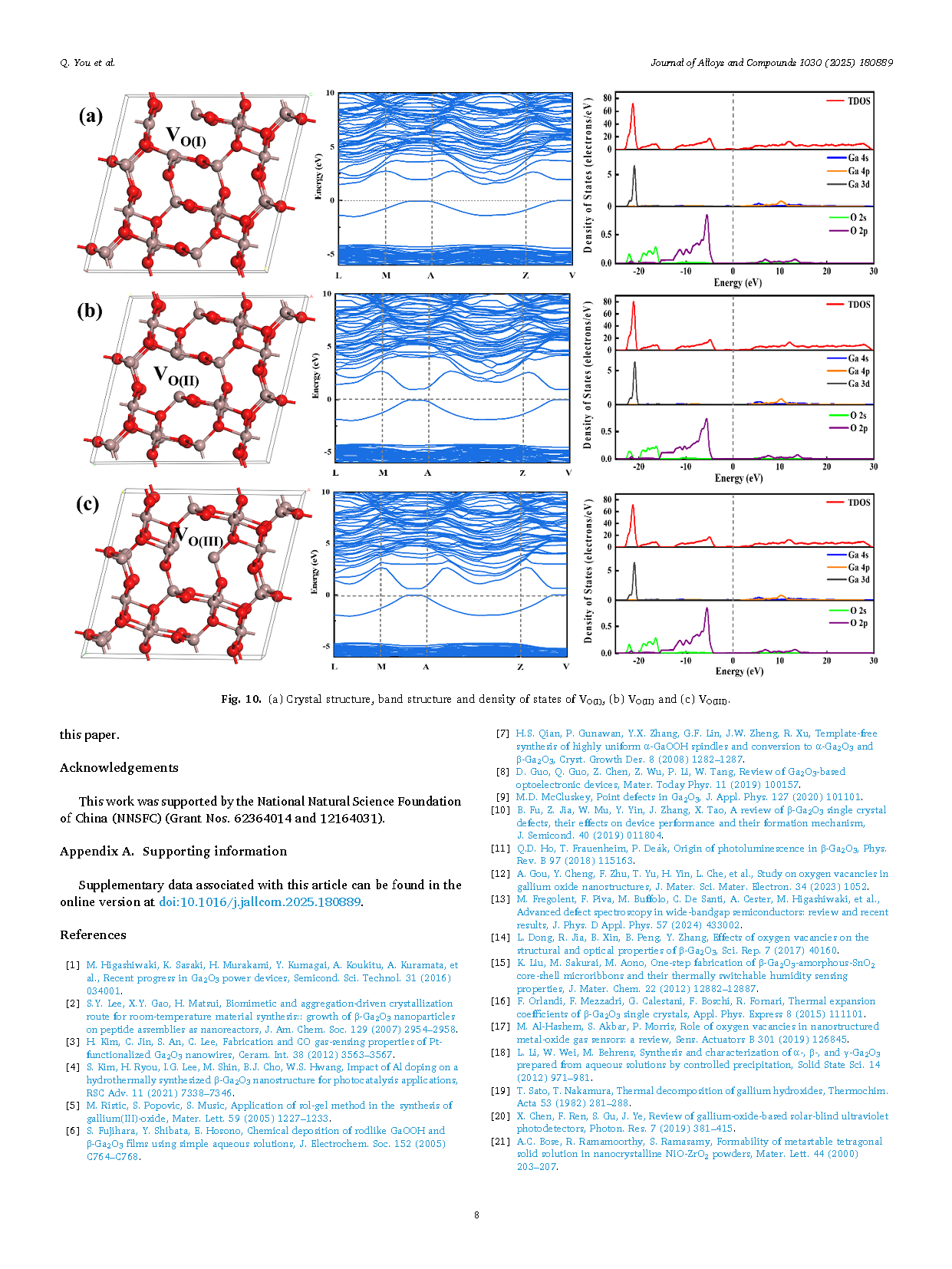
Gallium Oxide, especially in its stable β-phase (β-Ga2O3), has been extensively studied for its excellent opto-electronic properties, such as ultra-wide band gap (4.6 eV-4.9 eV), high breakdown electric field (8 MV/cm) and large Baliga’s figure of merit (BFOM, 3444). It is known that β-Ga2O3 polycrystalline powder of high purity, uniform grain size and adjustable morphology can be utilized for single crystal growth, gas sensors, photocatalysis and so on. There are in general two basic routes, i.e., solid state reaction and wet chemical method (e.g., sol-gel method, hydrothermal method, precipitation method), to prepare β-Ga2O3 polycrystalline powder. Among all the preparation techniques, the precipitation method (often followed by a post thermal treatment) has been proved to be a simplified and high-efficiency one by just setting only a few experimental conditions like gallium source choice, solution acid/base environment and reaction temperature. Usually, α-GaOOH precursor is first synthesized by the precipitation reaction between nitrates or other gallium salts and hydroxyls in solution. Then, β-Ga2O3 powder is obtained after calcination of α-GaOOH in air above 700 °C. Despite of the simple process, it remains difficult to accurately control the crystallization and morphology of the obtained β-Ga2O3 powder.
Abstract
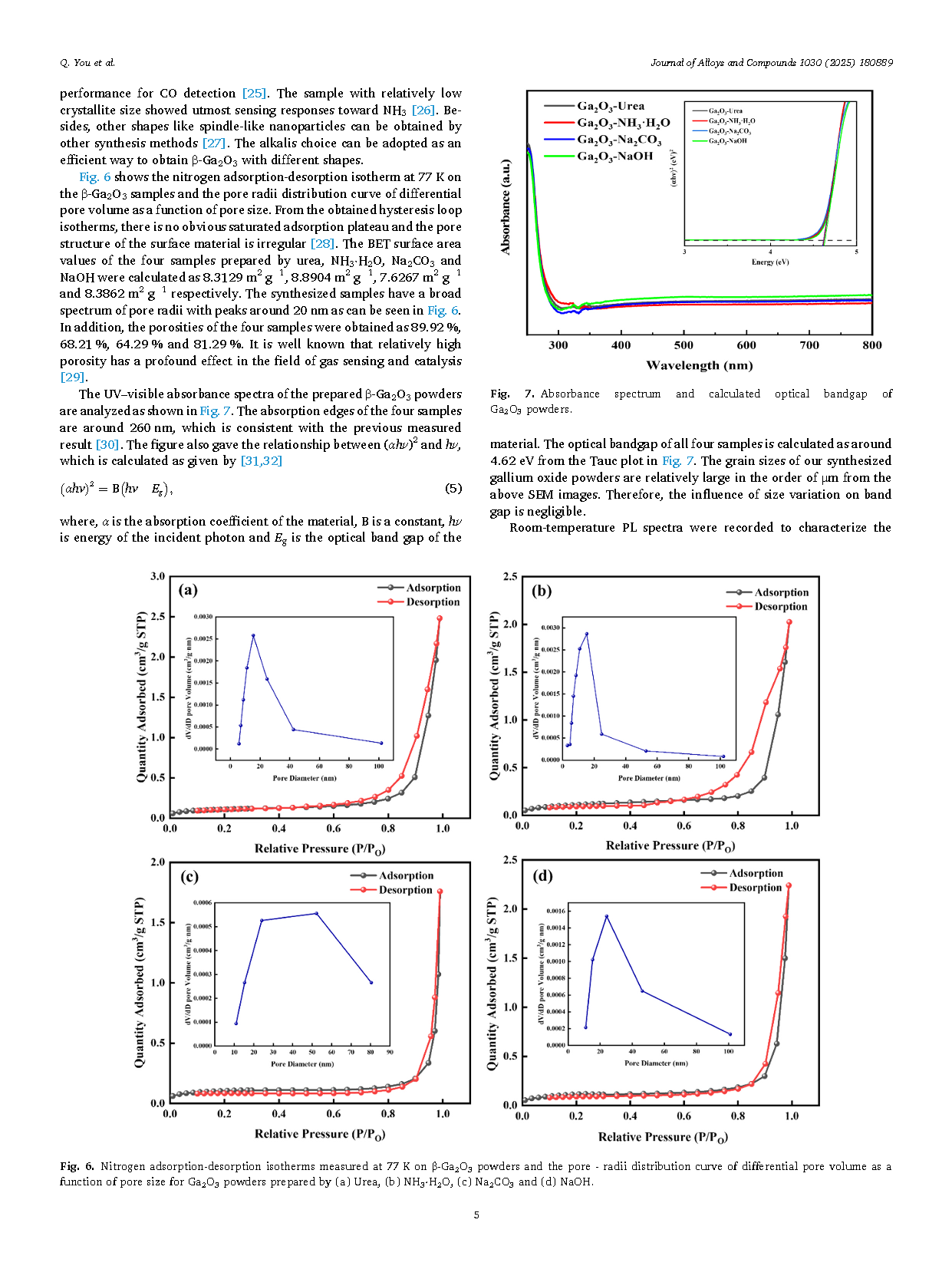
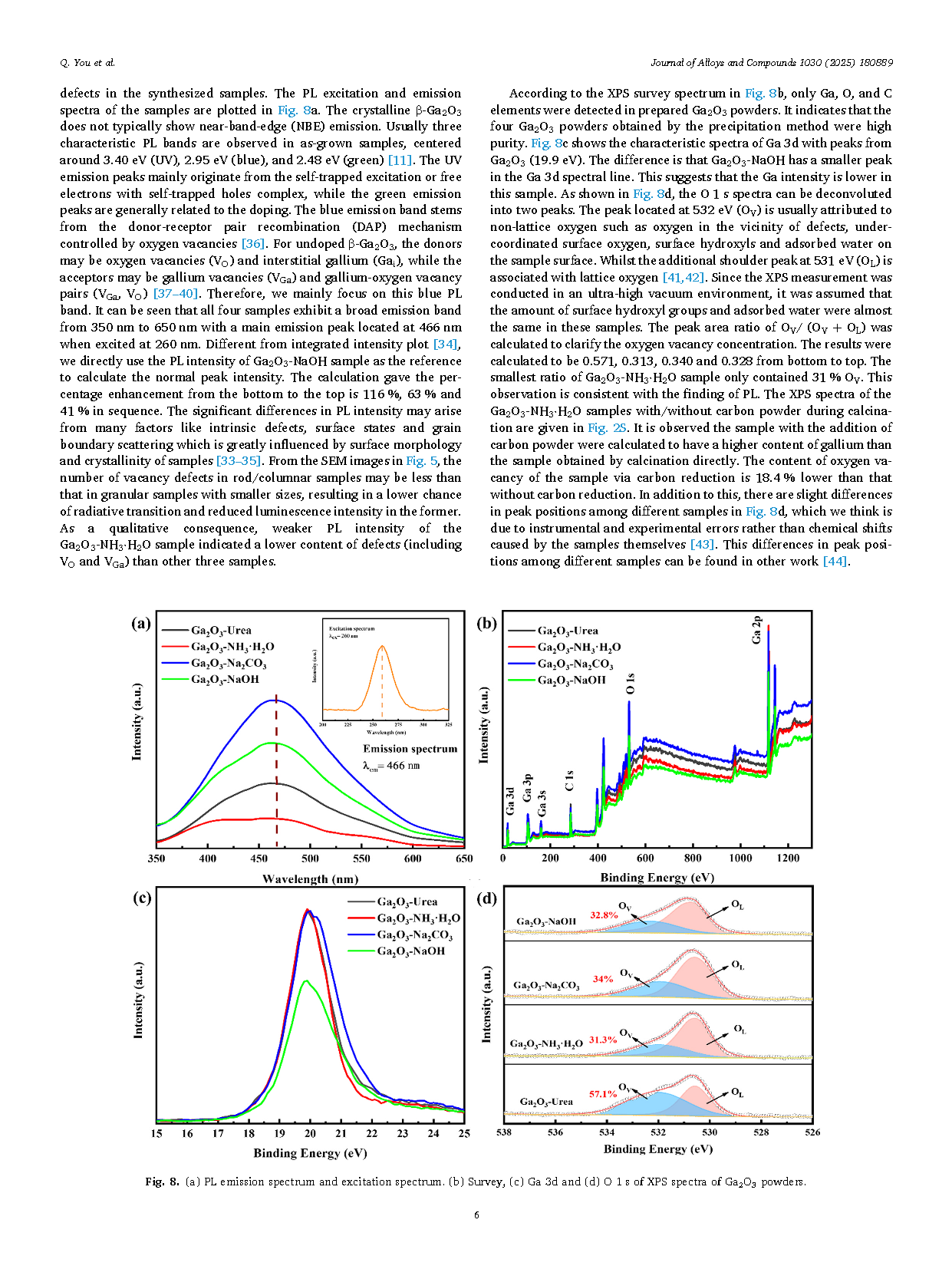
Controlling the crystallization of gallium oxide (Ga2O3) crystal as well as intrinsic defects (oxygen vacancies) are crucial steps in obtaining high-quality material and elucidating how defects affect semiconductor properties. Gallium oxide hydroxide (GaOOH) was firstly synthesized via the reaction of gallium nitrate (Ga(NO3)3) with different alkalis (Urea, NH3·H2O, Na2CO3 and NaOH) using the precipitation technique. Then, Ga2O3 powder was obtained by adding carbon powder as reducing agent at the calcination stage. The β-Ga2O3 powder prepared by NH3·H2O were found to have the best crystallization as analyzed by X-ray diffractometer (XRD) and scanning electron microscopy (SEM). Meanwhile, the photoluminescence (PL) and X-ray photoelectron spectroscopy (XPS) results combined with the first-principles calculation indicate that the Ga2O3 powder made by NH3·H2O also has the least intrinsic oxygen vacancies.
Highlights
● β-Ga2O3powders are prepared via a wet chemical method.
● The crystallization and morphology of β-Ga2O3powders are adjusted from alkalis choice.
● Oxygen vacancies are studied by PL, XPS and DFT calculation.
Conclusions
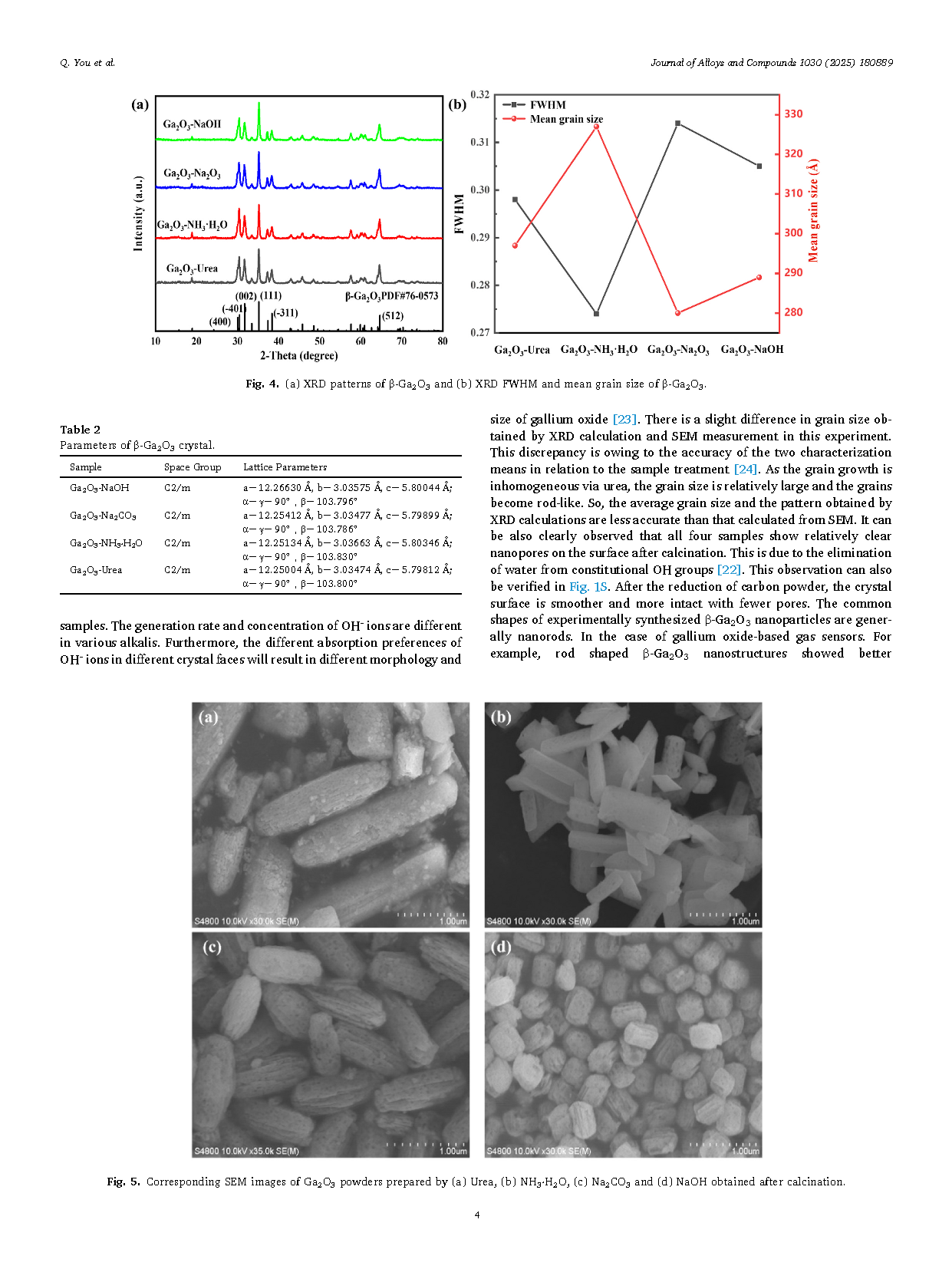
In summary, this paper gives the experimental procedure for the preparation of β-Ga2O3 powder using the precipitation method. As a result, the best crystallization of Ga2O3 powder was obtained by NH3·H2O. And the addition of carbon powder is helpful in improving the crystallization. It was also found that the sample prepared by NH3·H2O had the least amount of oxygen vacancies. Finally, we conclude that both crystallization and oxygen vacancies can be effectively controlled by the way of using different sources of hydroxide. The first-principles calculation results reveal that the oxygen vacancies at different locations have an effect on the bandgap structure. The results provide some references for the wet synthesis of Ga2O3 polycrystalline powders which can be found potential application in the field of gas detection.
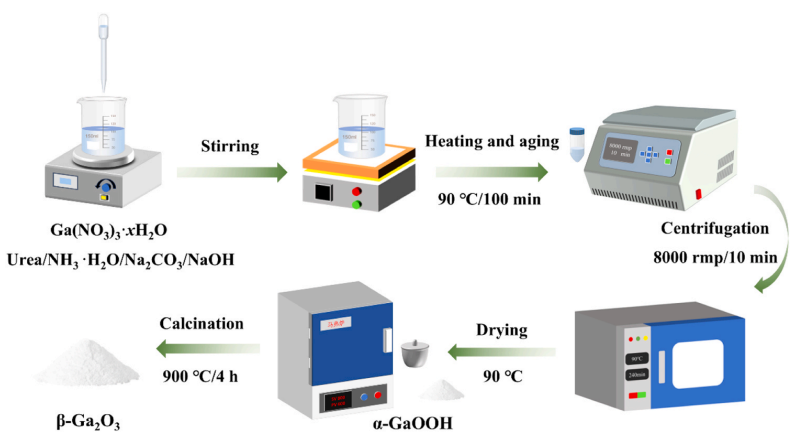
Fig. 1. Schematic diagram for preparation of Ga2O3 by precipitation technique.
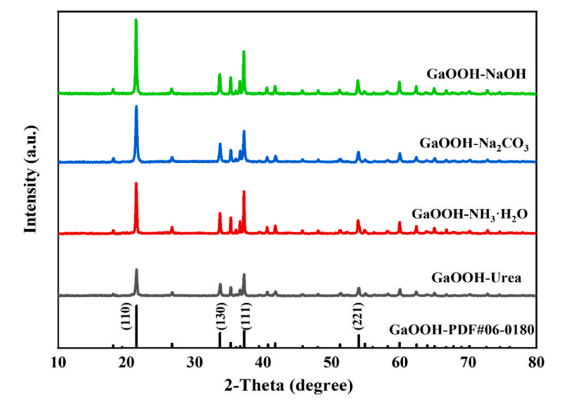
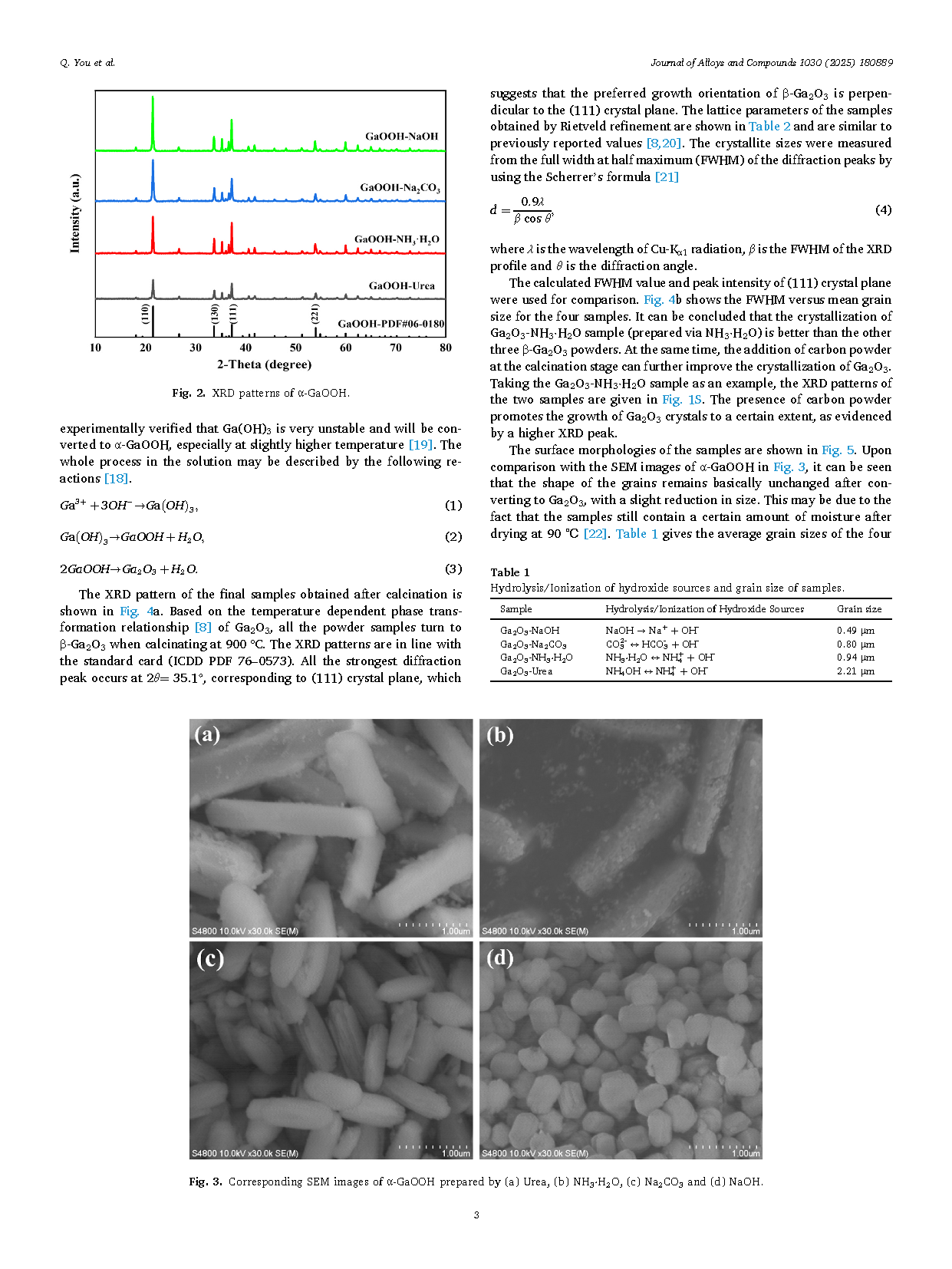
Fig. 2. XRD patterns of α-GaOOH.
DOI:
doi.org/10.1016/j.jallcom.2025.180889